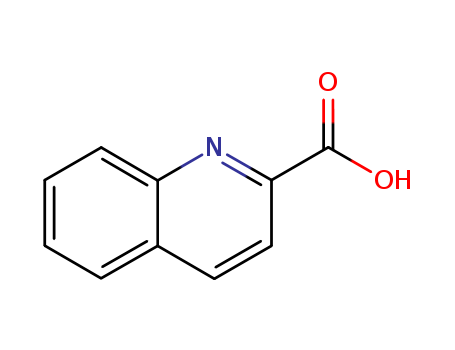

CasNo: 93-10-7
MF: C10H7NO2
Appearance: light brown needle-like crystalline powder
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 68, p. 1840, 1946 DOI: 10.1021/ja01213a045 |
|
Purification Methods |
Crystallise quinaldic acid from *C6H6 or AcOH. It is used for the estimation of many metals. The methyl ester has m 86-87o (from hexane) and pK25 1.76. [Chauduri et al. Frez Z Anal Chem 281 361 1976, Beilstein 22 H 71, 22 II 55, 22 III/IV 1149, 22/3 V 183.] |
|
Definition |
ChEBI: A quinolinemonocarboxylic acid having the carboxy group at the 2-position. |
|
General Description |
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers. |
InChI:InChI=1/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)/p-1
The oxidation of primary alcohols and al...
Prostate cancer (PC) is the most common ...
Quinoline derivatives are important natu...
We report the conversion of amides to ca...
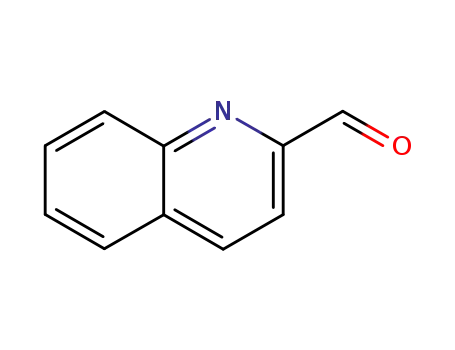
quinoline 2-carbaldehyde

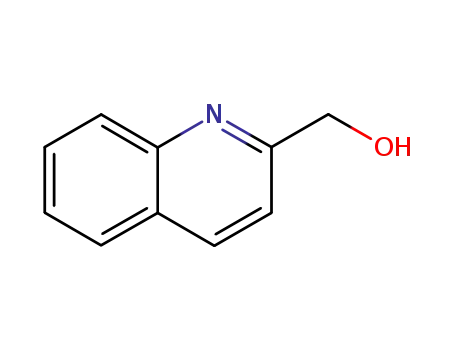
quinolin-2-ylmethanol

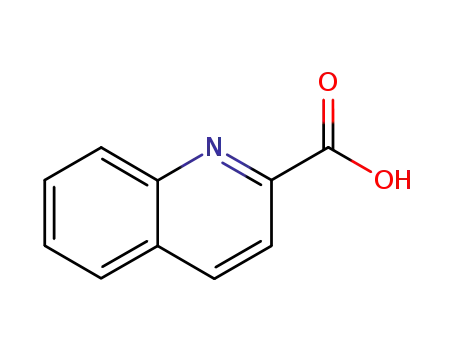
quinoline-2-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
|
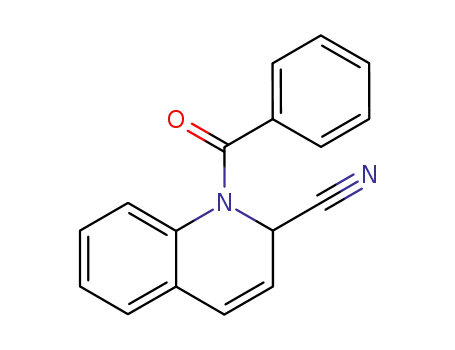
1-benzoyl-1,2-dihydro-quinoline-2-carbonitrile


quinoline-2-carboxylic acid

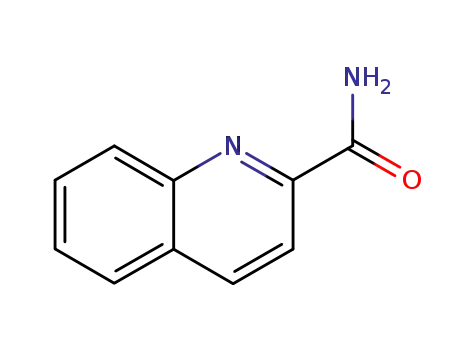
quinoline-2-carboxamide

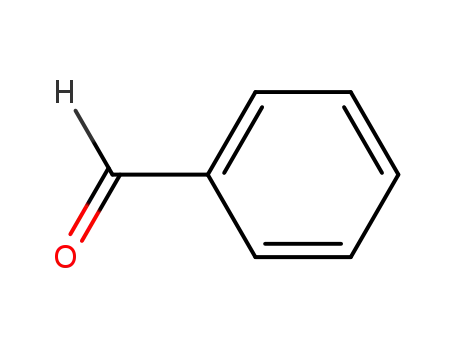
benzaldehyde
| Conditions | Yield |
|---|---|
|
With
formic acid;
at 20 ℃;
for 20h;
|
98% 82% 1% |
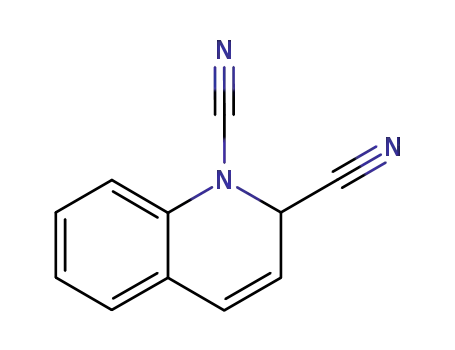
2H-quinoline-1,2-dicarbonitrile
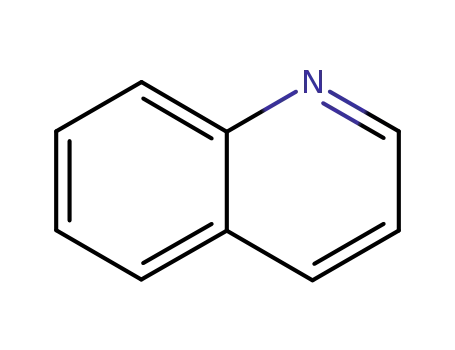
quinoline

hydrogen cyanide
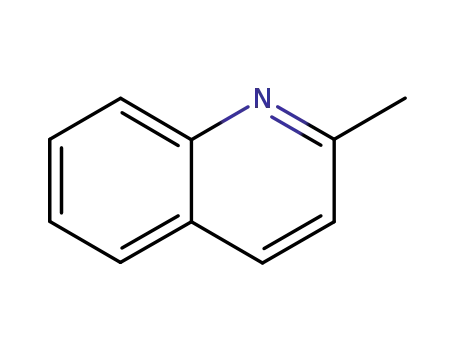
2-methylquinoline

1,1-diphenyl-1-(2-quinolyl)methanol
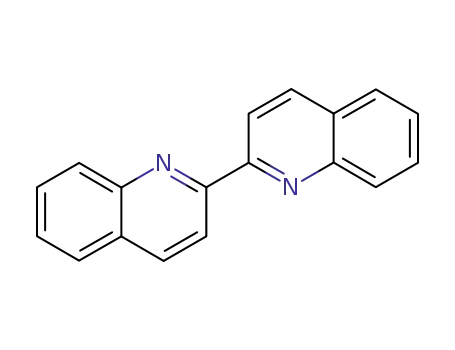
2,2'-biquinoline
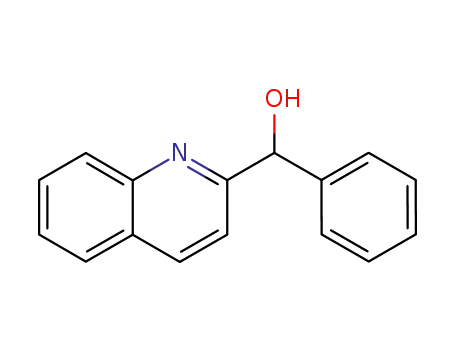
α-phenyl-2-quinolinemethanol
(4-methoxyphenyl)(quinoline-2-yl)methanone