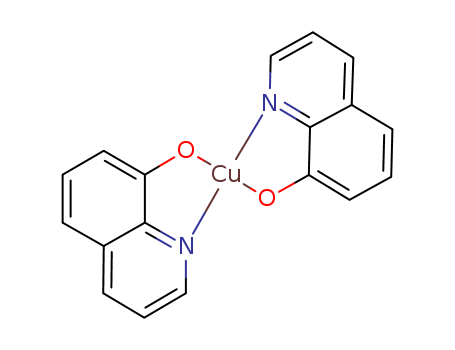

CasNo: 10380-28-6
MF: C18H12CuN2O2
Appearance: Yellow to greenish powder
|
Hazard |
Toxic by ingestion. Questionable carcinogen. |
|
Potential Exposure |
Fungicide and microbiocide. |
|
Shipping |
UN3077 Environmentally Hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Incompatibilities |
May form highly unstable acetylides. Decomposes on burning producing toxic and corrosive fumes including copper and nitrogen oxides |
|
Waste Disposal |
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Copper-containing wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. Details of copper recovery from a variety of industrial wastes have been published. If recovery is not feasible, the copper can be precipitated by the use of caustics and the sludge deposited in a chemical waste landfill. Recommendable Methods: Precipitation, solidification, landfill, discharge to sewer, & incineration. Peer-review: Precipitate copper with alkali, filter, solidify precipitate. (Do not use ammonia as alkali). Cation exchange will allow recovery of copper. Eluate from cation exchanger can be passed through anion exchanger to remove (or reduce) naphthenic acid content. Exhausted ion exchange resins can be landfilled. (Peer-review conclusions of an IRPTC expert consultation) |
InChI:InChI=1/2C10H7NO3.Cu/c2*12-8-3-1-2-6-4-5-7(10(13)14)11-9(6)8;/h2*1-5,12H,(H,13,14);/q;;+2/p-2
8-Hydroxyquinolines (8HQs) are a family ...
A simple and sensitive conductometric me...
Bis(8-quinolinolato)copper(II) complex i...
In present investigation nanocrystalline...
A ternary complex, (D-alaninato)(L-isole...
Insensitivity to platinum, either throug...
We describe the construction of syntheti...
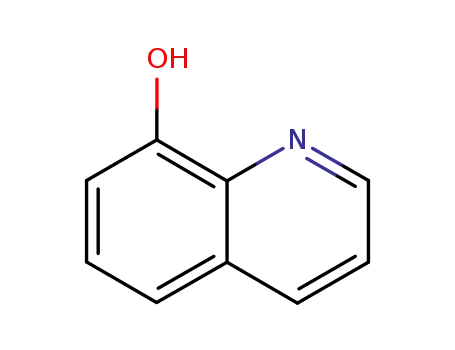
8-quinolinol

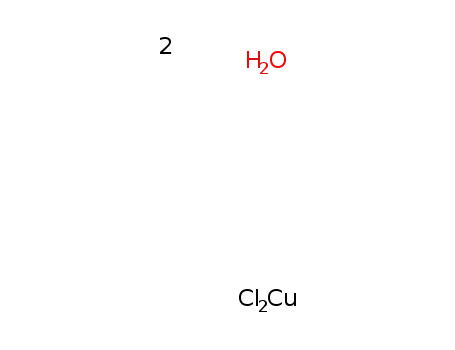
copper(II) choride dihydrate

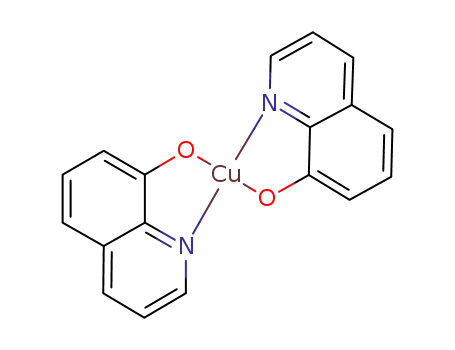
bis(8-hydroxyquinolato)copper(II)
| Conditions | Yield |
|---|---|
|
In
methanol; water;
for 0.5h;
|
77% |
|
With
potassium hydroxide;
In
ethanol;
Reflux;
|
71.21% |
|
With
sodium acetate;
In
ethanol; acetic acid;
addn. of an excess of quinoline in EtOH at 50°C to a soln. of Cu-salt and sodium acetate in aq. acetic acid, briefly warming to 80-90°C, filtn., washing with hot H2O, hydrated complex is heated to 130°C for several h; elem. anal.;
|

8-quinolinol

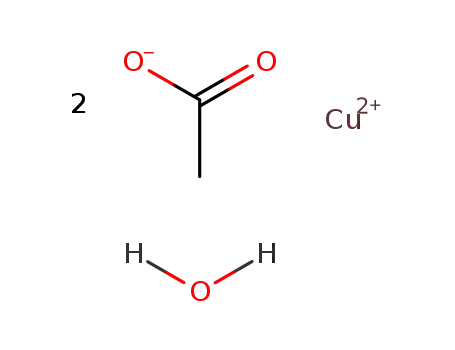
copper(II) acetate monohydrate


bis(8-hydroxyquinolato)copper(II)
| Conditions | Yield |
|---|---|
|
In
neat (no solvent, solid phase);
solid state react. of CuAc2 with 8-hydroxyquinoline; elem. anal.;
|
85% |
|
In
not given;
Cu(CH3COO)2*H2O was mixed with ligand; dried at 110°C for 12 h;
|
|
|
In
tetrahydrofuran;
for 2h;
Reflux;
|

8-quinolinol

copper
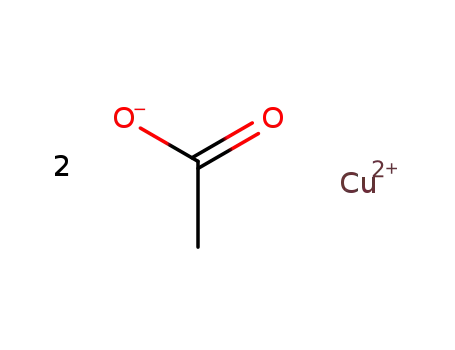
copper diacetate

copper(II) acetate monohydrate
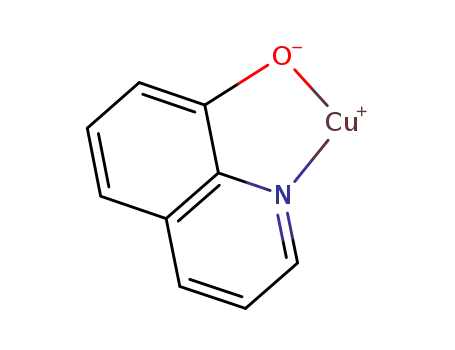
Cu(8-HQ)
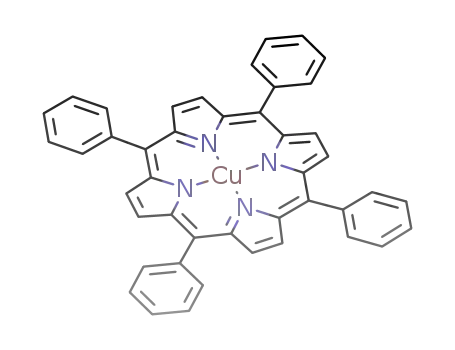
(tetraphenylporphyrin)copper(II)