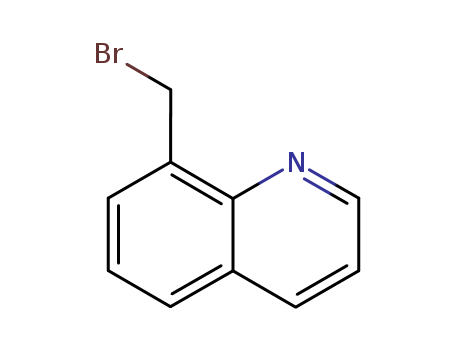

CasNo: 7496-46-0
MF: C10H8BrN
InChI:InChI=1/C10H8BrN/c11-7-9-4-1-3-8-5-2-6-12-10(8)9/h1-6H,7H2
The new alkaloid sibiridine was isolated...
A new 7,16-bis(quinolin-8-ylmethyl)-1,4,...
Two macrocyclic dioxotetraamine ligands ...
Two bisquinoline derivatives, N,N'-bis(2...
Disclosed herein are compounds of formul...
A general method for the oxidative subst...
An operationally simple and metal-free p...
Five bis(quinolylmethyl)-(1H-indolylmeth...
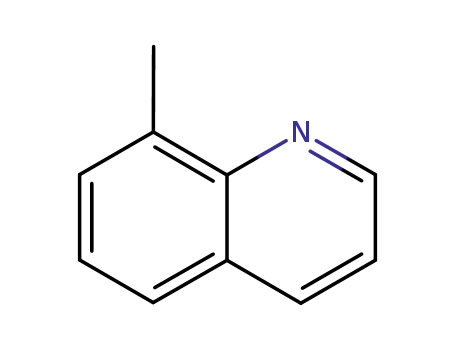
8-methylquinoline

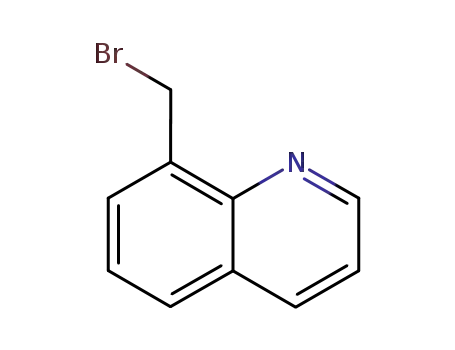
8-bromomethyl-quinoline
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
for 12h;
Heating;
|
88% |
|
With
N-Bromosuccinimide; Perbenzoic acid;
In
tetrachloromethane;
for 6h;
Heating;
|
85% |
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
tetrachloromethane;
for 3h;
Reflux;
|
82% |
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
tetrachloromethane;
for 36h;
Heating;
|
80% |
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
tetrachloromethane;
for 2h;
|
75% |
|
With
N-Bromosuccinimide; Perbenzoic acid;
In
tetrachloromethane;
for 3h;
Heating;
|
58% |
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
for 6h;
Reflux;
Inert atmosphere;
|
58% |
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
cyclohexane;
for 10h;
Inert atmosphere;
Reflux;
|
46% |
|
With
tetrachloromethane; N-Bromosuccinimide; dibenzoyl peroxide;
Irradiation.mit UV-Licht;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
Heating;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
for 6h;
Heating;
|
|
|
With
N-Bromosuccinimide;
In
tetrachloromethane;
at 60 ℃;
for 1h;
|
0.5 g |
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane; chloroform;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane; water;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
1,2-dichloro-ethane;
for 30h;
Reflux;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide;
In
tetrachloromethane;
Inert atmosphere;
Schlenk technique;
Reflux;
|

8-methylquinoline

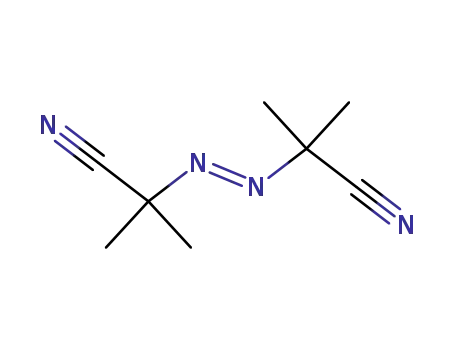
azobisisobutyronitrile


8-bromomethyl-quinoline
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
tetrachloromethane;
|

8-methylquinoline
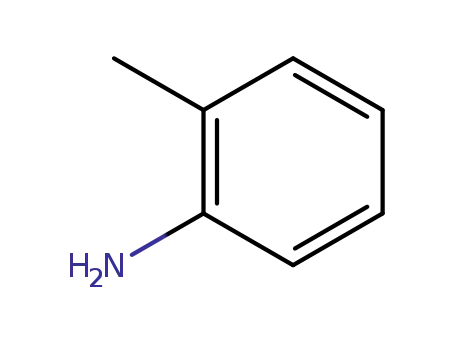
o-toluidine

azobisisobutyronitrile
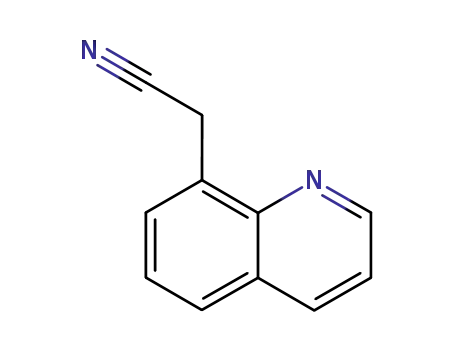
[8]quinolyl-acetonitrile
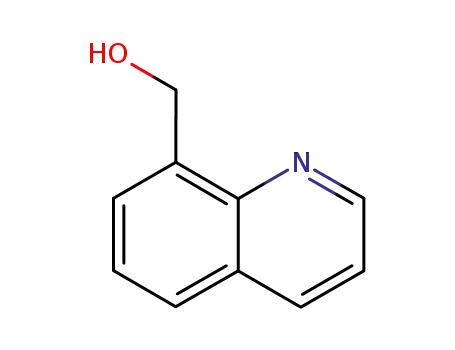
8-hydroxymethylquinoline
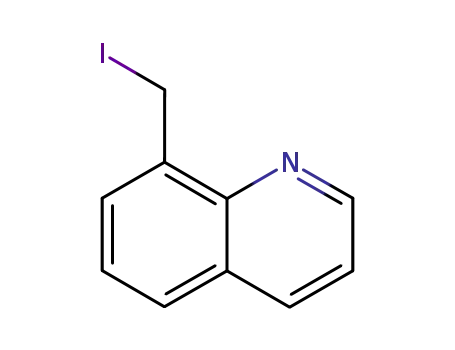
8-iodomethylquinoline
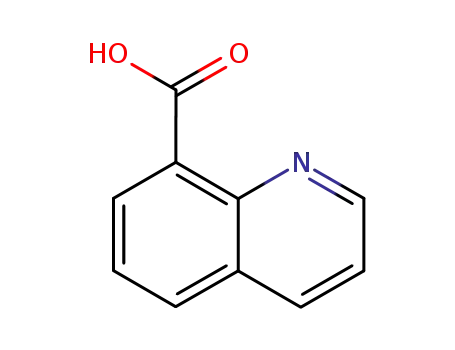
8-carboxyquinoline