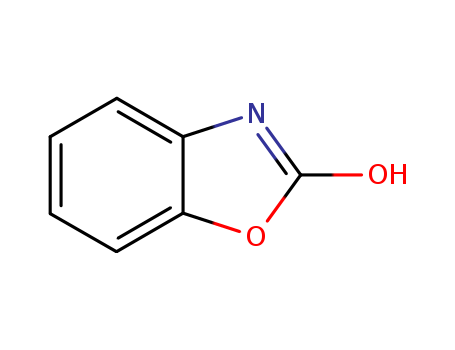

CasNo: 59-49-4
MF: C7H5NO2
Appearance: light beige to brown-grey powder
|
Preparation |
2-benzoxazolinone synthesis method: add o-aminophenol, urea and solvent chlorobenzene into the reaction kettle, protect with nitrogen, the reaction temperature is 50~132 ℃, the reaction time is 6h, after the reaction is completed, it is cooled with ice brine, crystallized, Filter the finished product. It is also possible to use o-aminophenol to react with phosgene in the solvent chlorobenzene, quickly pass phosgene within 20 ~ 40 ° C, then heat up to 100 ~ 130 ° C, and then pass phosgene at a lower speed, after the reaction is completed, pass nitrogen to catch up. Phosgene, after work-up, can also obtain 2-benzoxazolinone. |
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 6, p. 123, 1969 DOI: 10.1002/jhet.5570060124Synthetic Communications, 34, p. 735, 2004 DOI: 10.1081/SCC-120027722Synthesis, p. 1032, 1983 DOI: 10.1055/s-1983-30616 |
|
General Description |
2-Benzoxazolinone is a phytoanticipin and its biotransformation by endophytic fungi isolated from Aphelandra tetragona was studied. 2-Benzoxazolinone is a natural chemical produced by rye (Secale cereale) and has strong phytotoxic properties. |
InChI:InChI=1/C7H5NO2/c9-7-8-5-3-1-2-4-6(5)10-7/h1-4H,(H,8,9)
-
A novel approach was proposed to synthes...
-
-
A new class of 2-benzoxazolinone derivat...
Aminolysis of carbamic esters, a model o...
Catalytic carbon-nitrogen bond formation...
A new series of 1,3-benzoxazol-2(3H)-one...
Catalysts as the dynamo of chemical reac...
Novel Ru(II) complexes of Shiff base der...
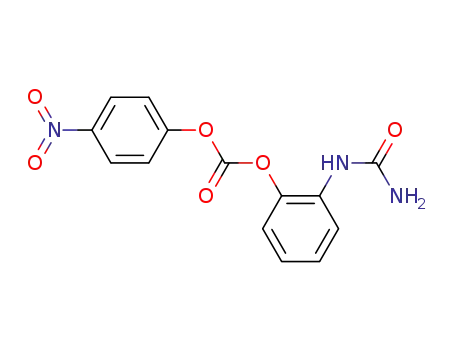
p-nitrophenyl 2-ureidophenylcarbonate

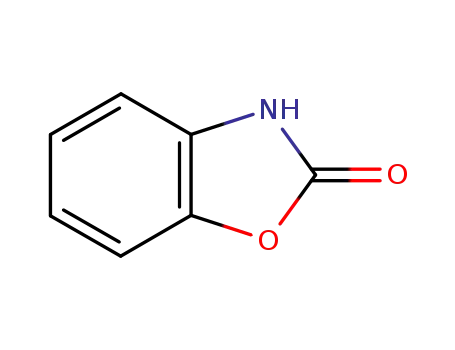
2-Benzoxazolinone

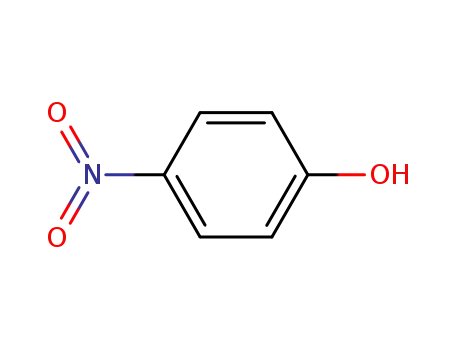
4-nitro-phenol

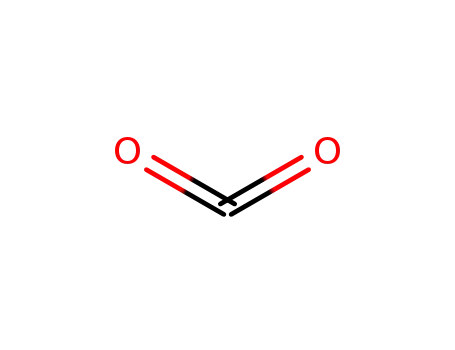
carbon dioxide
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
acetonitrile;
at 30 ℃;
Rate constant;
|
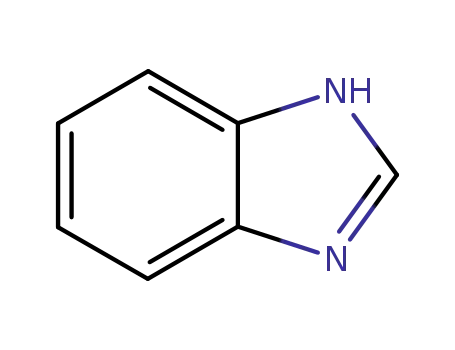
benzoimidazole


2-Benzoxazolinone

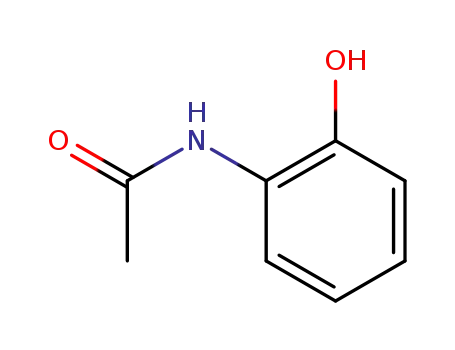
2-(acetylamino)phenol

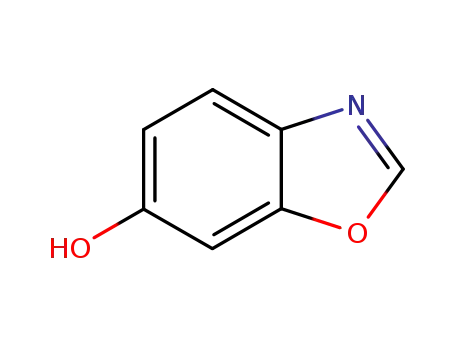
6-hydroxybenzoxazole

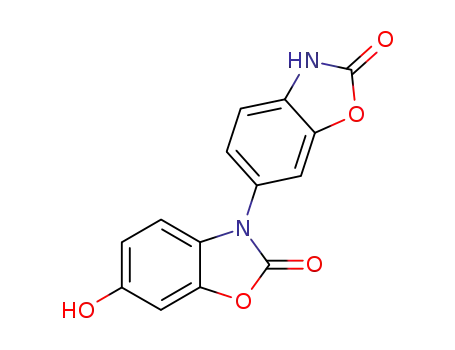
6-hydroxy-3-(2'-oxo-2',3'-dihydrobenzoxazol-6'-yl)benzoxazol-2(3H)-one
| Conditions | Yield |
|---|---|
|
With
lead(IV) acetate; acetic acid;
at 80 ℃;
for 8h;
Further byproducts given;
|
6% 9% 29% 2.5% |
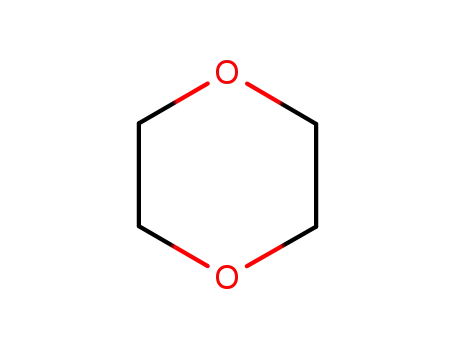
1,4-dioxane
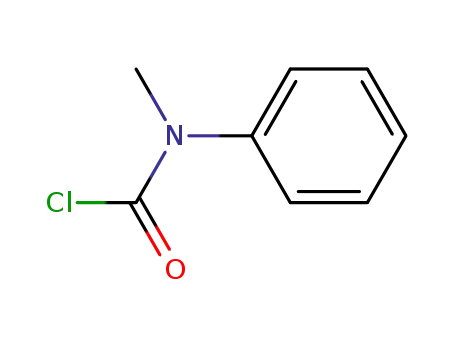
N-phenyl-N-methylcarbamoyl chloride
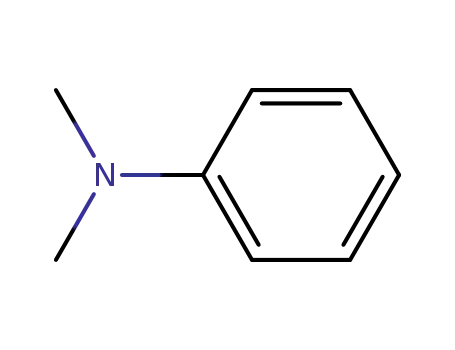
N,N-dimethyl-aniline
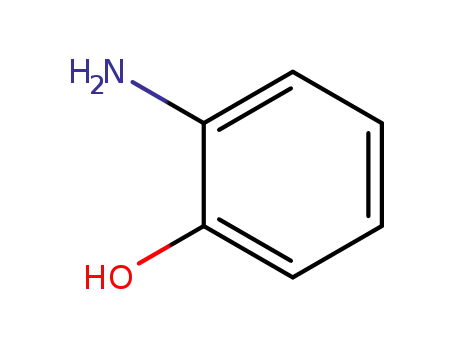
2-amino-phenol
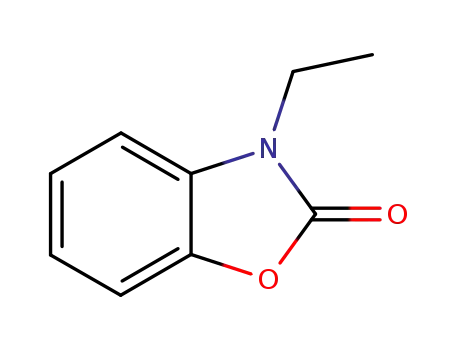
N-ethyl benzoxazol-2-one
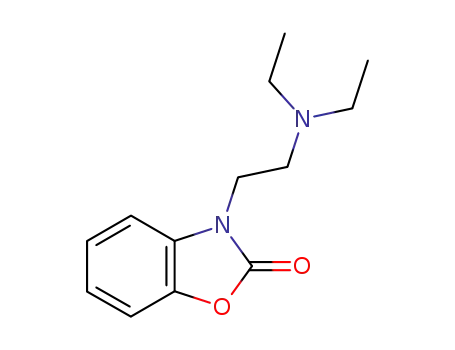
3-<2-Diaethylaminoaethyl>-2-benzoxazolin
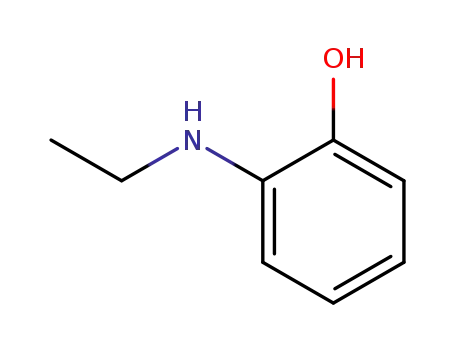
2-ethylaminophenol
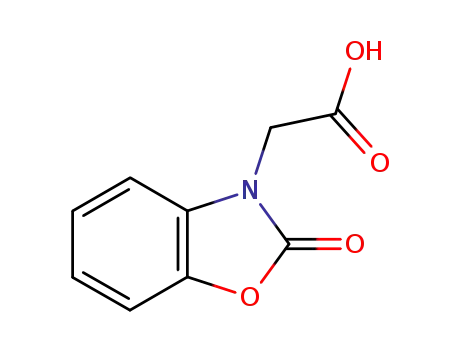
2-[2-oxo-benzo[d]oxazole-3(2H)-yl]acetic acid