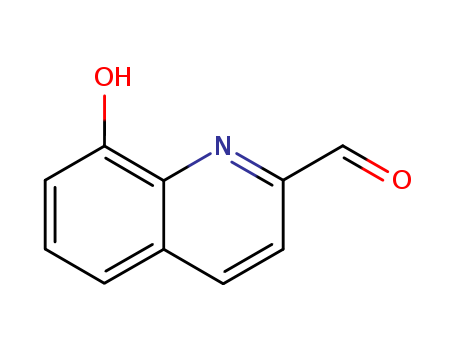

CasNo: 14510-06-6
MF: C10H7NO2
Appearance: Yellow crystalline powder
|
General Description |
8-Hydroxy-2-quinolinecarboxaldehyde can be prepared from 2-methylquinolin-8-ol via oxidation using selenium dioxide. |
InChI:InChI=1/C10H7NO2/c12-6-8-5-4-7-2-1-3-9(13)10(7)11-8/h1-6,13H
A novel fluorescent probe based on hydro...
A new Cu2+-selective probe was developed...
The reaction of a new hexadentate Schiff...
Methionine aminopeptidase 1 of Leishmani...
Novel lavendamycin analogues with variou...
Herein, we report a comprehensive study ...
A series of novel 8-substituted-N-(4-sul...
A rhodamine-based sensor has been develo...
Starting from a Schiff base ligand conta...
In this study, a highly selective fluore...
A rhodamine-based sensor (1) has been de...
An acyclic hexadentate oxine-derived che...
A new quinoline-indolium-based chemical ...
A new fluorescence turn-on chemosensor 1...
The reaction of 8-quinolinol-2-carboaldo...
A tripodal ligand based on the 8-hydroxy...
[44/47Sc]Sc3+, [68Ga]Ga3+, and [111In]In...
Uranium-230 is an α-emitting radionuclid...
A new tripodal chelator 2,2′,2′′-((1E,1′...
Abstract: A new series of N-benzylpiperi...
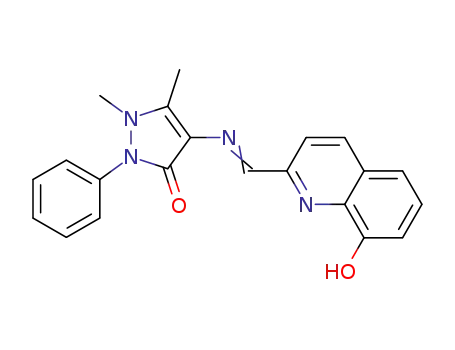
4-(((8-hydroxyquinolin-2-yl)methylene)amino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one

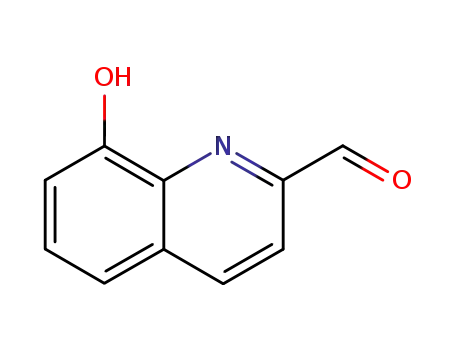
2-formyl oxine

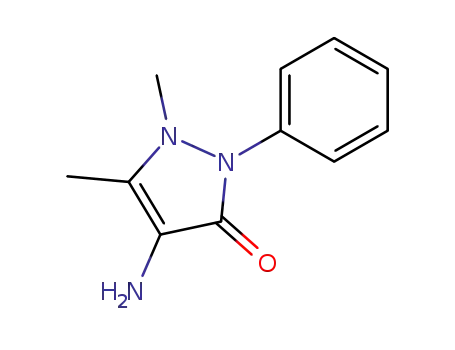
4-amino-2,3-dimethyl-1-phenylpyrazolin-5-one
| Conditions | Yield |
|---|---|
|
With
copper(II) perchlorate hexahydrate; water;
|
2-methyl-8-quinolinol


2-formyl oxine
| Conditions | Yield |
|---|---|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
for 24h;
|
95% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
at 80 ℃;
for 24h;
Inert atmosphere;
|
90% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
at 60 ℃;
for 12.5h;
Reflux;
|
86% |
|
With
selenium(IV) oxide; formaldehyd;
In
1,4-dioxane;
Reflux;
|
86% |
|
With
selenium(IV) oxide;
at 80 ℃;
|
82% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
|
82% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 95 ℃;
for 24h;
Reflux;
|
80% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 95 ℃;
for 24h;
Reflux;
|
80% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 60 - 120 ℃;
for 2.5h;
|
79% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 24h;
Reflux;
|
74.5% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 24h;
Reflux;
|
74.5% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
for 12h;
|
70% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
for 24h;
Inert atmosphere;
|
65% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 95 ℃;
for 21h;
Inert atmosphere;
|
65% |
|
With
selenium(IV) oxide; tetrabutyl ammonium fluoride; tert-butyldimethylsilyl chloride;
|
64% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
Heating;
|
61% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 50 - 80 ℃;
for 19h;
|
61% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
at 60 ℃;
for 3h;
Reflux;
|
61.3% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
for 24h;
Reflux;
|
59.9% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 100 ℃;
for 3h;
|
56.9% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
at 80 ℃;
for 24h;
Inert atmosphere;
|
53.6% |
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
at 70 - 100 ℃;
for 3h;
|
52% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 85 ℃;
for 8h;
|
51% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 70 ℃;
for 8h;
Inert atmosphere;
|
50% |
|
With
selenium(IV) oxide;
In
water; ethyl acetate;
for 48h;
Reflux;
|
46% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
for 18h;
|
45% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 60 - 80 ℃;
for 10h;
Inert atmosphere;
|
40.5% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 50 - 80 ℃;
for 20h;
|
38% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 50 - 80 ℃;
for 20h;
|
38% |
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 50 - 80 ℃;
for 20h;
|
38% |
|
Multi-step reaction with 3 steps
1: ethanol; KOH-solution
2: selenium dioxide; water; dioxane / 100 °C
3: water; hydrochloric acid
With
1,4-dioxane; hydrogenchloride; potassium hydroxide; selenium(IV) oxide; ethanol; water;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 20h;
Inert atmosphere;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 20h;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 24h;
Reflux;
|
|
|
Multi-step reaction with 3 steps
1: 1H-imidazole / dichloromethane / 12 h / 20 °C / Inert atmosphere
2: selenium(IV) oxide / 1,4-dioxane / 1.5 h / 90 °C / Inert atmosphere
3: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
With
1H-imidazole; selenium(IV) oxide; tetrabutyl ammonium fluoride;
In
tetrahydrofuran; 1,4-dioxane; dichloromethane;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
Reflux;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 ℃;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
Reflux;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
for 24h;
Reflux;
|
|
|
With
selenium(IV) oxide;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 70 ℃;
Inert atmosphere;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane; water;
Reflux;
|
|
|
Multi-step reaction with 2 steps
1: 5 h / 138 °C
2: selenium(IV) oxide / 1,4-dioxane / 7 h / 75 - 80 °C
With
selenium(IV) oxide;
In
1,4-dioxane;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 110 ℃;
for 12h;
|
|
|
With
selenium(IV) oxide;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
|
|
|
With
pyridine; selenium(IV) oxide;
In
1,4-dioxane; water;
at 105 ℃;
|
|
|
With
selenium(IV) oxide;
Schlenk technique;
Inert atmosphere;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
|
|
|
With
selenium(IV) oxide;
In
1,4-dioxane;
at 80 - 90 ℃;
for 18h;
Inert atmosphere;
|
|
|
With
selenium(IV) oxide; water;
In
1,4-dioxane;
|
8-benzyloxyquinolin-2-yl-carboxaldehyde

2-methyl-8-quinolinol
2-methyl-8-(benzyloxy)quinoline
1,4-dioxane
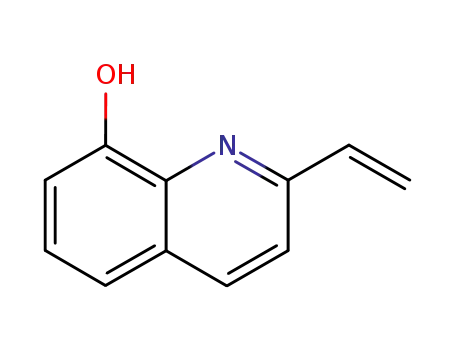
2-vinyl-8-quinolinol
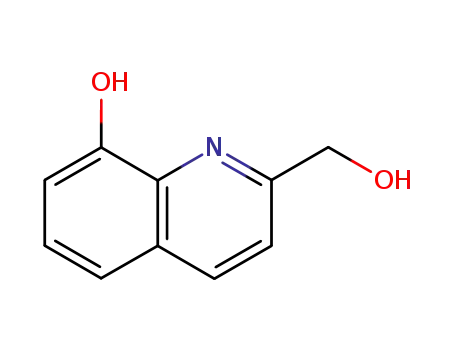
2-hydroxymethyl-8-hydroxyquinoline
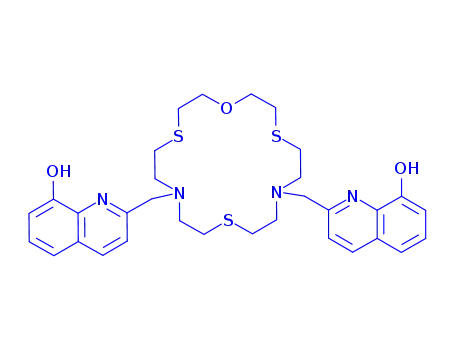
7,13-bis(8-hydroxyquinolin-2-ylmethyl)-7,13-diaza-4,10,16-trithia-1-oxacyclooctadecane
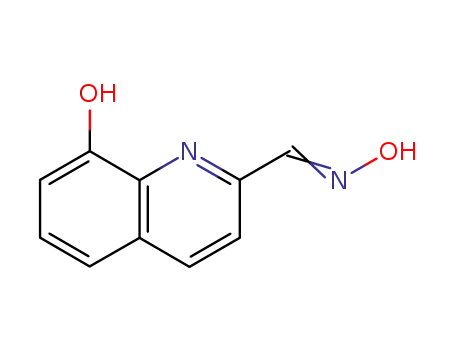
8-hydroxyquinoline-2-carbaldehyde oxime