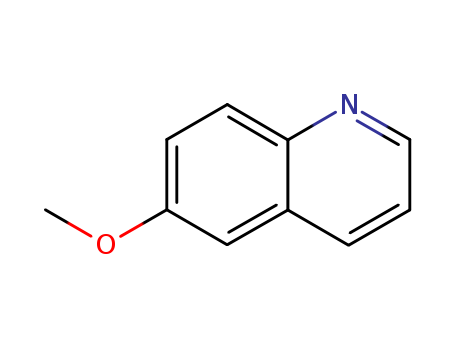

CasNo: 5263-87-6
MF: C10H9NO
Appearance: colorless to light yellow liquid
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 68, p. 1584, 1946 DOI: 10.1021/ja01212a062 |
|
Definition |
ChEBI: An aromatic ether that is quinoline substituted at position 6 by a methoxy group. |
InChI:InChI=1/C10H9NO/c1-12-9-4-5-10-8(7-9)3-2-6-11-10/h2-7H,1H3
Ruthenium trichloride hydrate combined w...
-
A highly enantioselective addition of hy...
-
The identification of new photocatalytic...
The invention provides a method for real...
The epoxidation of olefin as a strategy ...
We report herein an unprecedented combin...
Herein, we disclose a highly chemoselect...
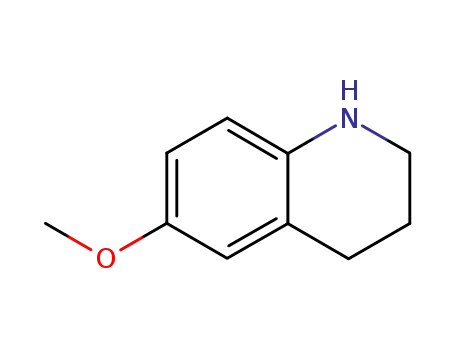
6-methoxy-1,2,3,4-tetrahydroquinoline

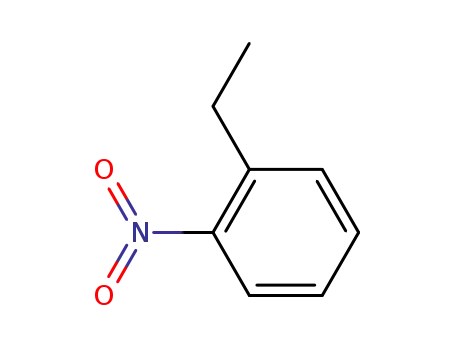
2-nitro(ethylbenzene)

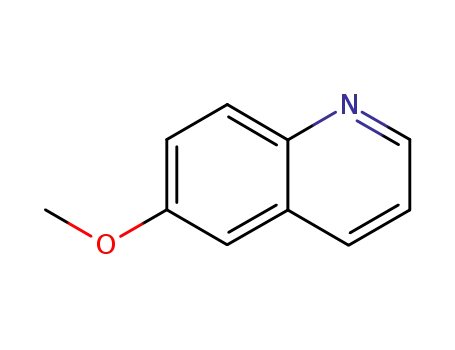
6-methoxy quinoline

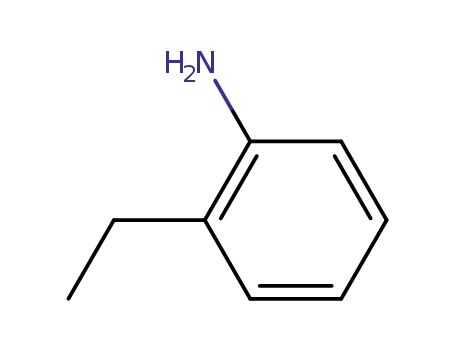
ortho-ethylaniline
| Conditions | Yield |
|---|---|
|
With
nickel-nitrogen-doped carbon framework;
In
water;
at 145 ℃;
for 18h;
Inert atmosphere;
Sealed tube;
Green chemistry;
|
52% 48% |
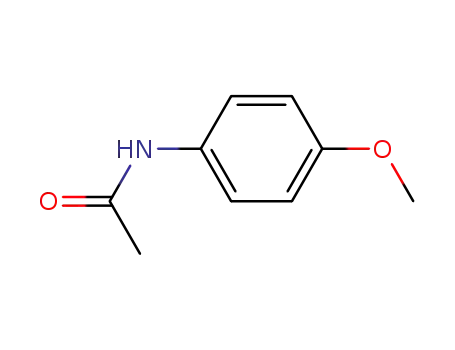
4-methoxyacetanilide

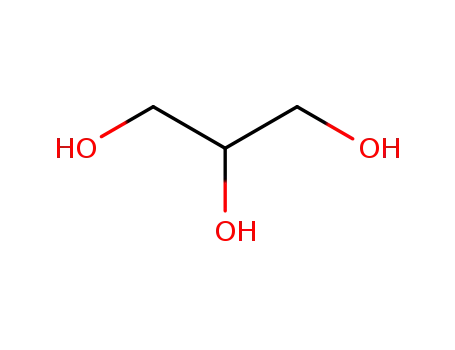
glycerol

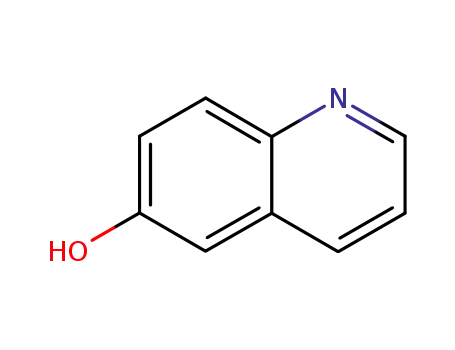
6-hydroxyquinoline


6-methoxy quinoline
| Conditions | Yield |
|---|---|
|
With
para-methoxynitrobenzene; sulfuric acid; boric acid;
at 135 ℃;
|

6-hydroxyquinoline
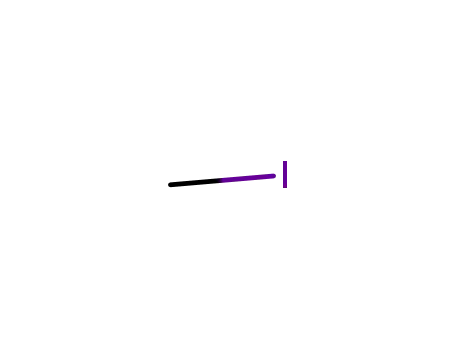
methyl iodide

4-methoxyacetanilide

glycerol
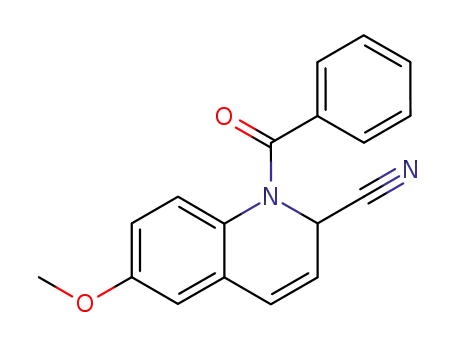
1-benzoyl-6-methoxy-1,2-dihydro-quinoline-2-carbonitrile
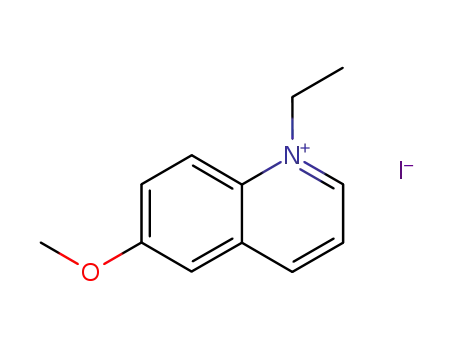
1-ethyl-6-methoxyquinolin-1-ium iodide
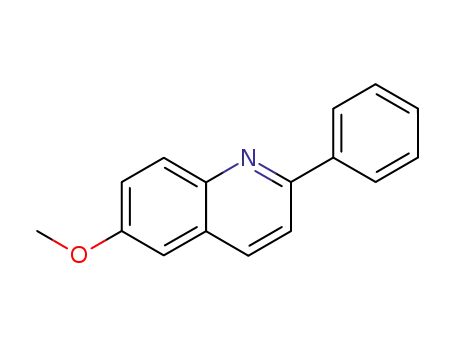
2-phenyl-6-methoxyquinoline
3-benzyl-6-methoxy-quinoline