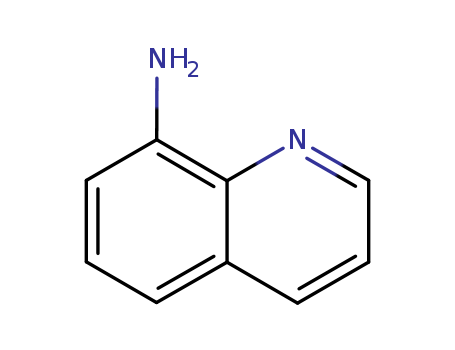

CasNo: 578-66-5
MF: C9H8N2
Appearance: green to beige-brown crystalline powder
|
Preparation |
Synthesis of 8-aminoquinoline: Under an ice bath, sulfuric acid (2.0 mL) was added onto quinoline (5 mmol, 1.0 equiv) then 65% nitric acid (3.0 equiv) were added dropwise and stirred for 4h at rt. The mixture was poured into the ice water and neutralized with NaOH; and then extracted with dichloromethane. After dried over Na2SO4 and evaporated in vacuo, used next step without purification.Mixture of nitroquinolines and 5% Pd/C was solved in ethanol and suspension was saturated with hydrogen gas under atmospheric pressure at 40°C until the starting material was consumed. 2 h later, the mixture was filtered and evaporated. The crude product was purified by silica gel column chromatography, eluting with EtOAc in hexanes to yield the desired 8-aminoquinoline is isolated as a brown solid in a yield of 32%.Obtained as a brown solid (231 mg, 32%); 1H NMR (500 MHz, CDCl3) δ 8.71 – 8.60 (m, 1H), 7.93 (d, J = 8.2 Hz, 1H), 7.29 – 7.16 (m, 2H), 7.02 (d, J = 8.1 Hz, 1H), 6.80 (d, J = 7.4 Hz, 1H), 4.89 (s, 2H). |
|
Safety Profile |
Human mutation data reported.When heated to decomposition it emits toxic fumes ofNOx. |
|
Purification Methods |
8-Aminoquinoline crystallises from EtOH, ligroin, octane or H2O, and complexes with metals. [Beilstein 22 III/IV 4708, 22/10 V 316.] |
|
General Description |
8-Aminoquinoline fluoresce moderately to weakly in low dielectric media but not in strongly hydrogen-bonding or acidic aqueous media. The reaction of 8-aminoquinoline with chromium (III), manganese (II), iron (II) and (III), cobalt (II), nickel (II), copper (II), zinc (II), cadmium (II) and platinum (II) salts has been studied. |
InChI:InChI=1/C9H8N2/c10-8-5-1-3-7-4-2-6-11-9(7)8/h1-6H,10H2
A negative feedback loop that relies on ...
Ligand-assisted synthesis of metal nanop...
The hydrogenation of heterocyclic nitroa...
Transition metal catalysis that utilizes...
Herein, we developed a renewable carbon-...
The vicarious nucleophilic substitution ...
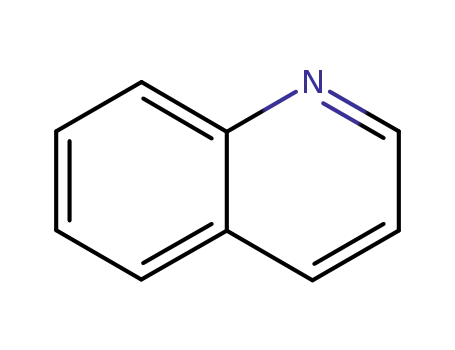
quinoline

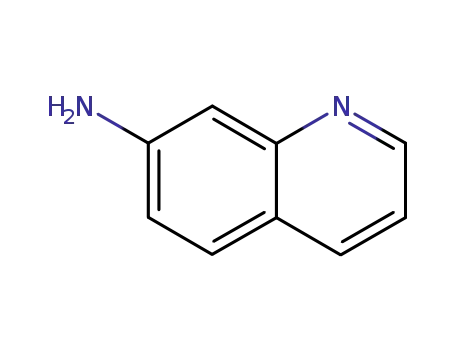
quinolin-7-ylamine

6-aminoquinoline

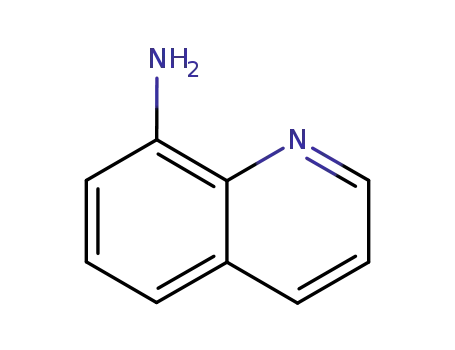
8-amino quinoline

5-Aminoquinoline
| Conditions | Yield |
|---|---|
|
quinoline;
With
tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; trifluorormethanesulfonic acid; C8H9F3NO3S(1+)*CF3O3S(1-);
In
acetonitrile;
at 23 - 25 ℃;
for 48h;
Sealed tube;
Irradiation;
With
N-butylamine;
In
acetonitrile;
at 40 ℃;
for 12h;
regioselective reaction;
|
32% 8% 8% 12% |
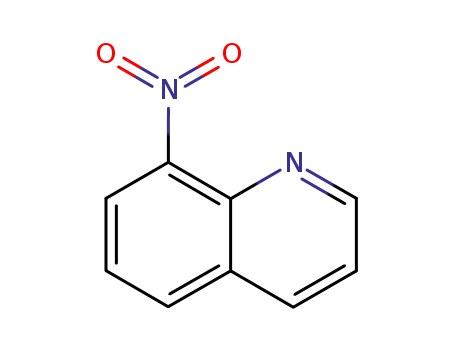
8-nitroquinoline


8-amino quinoline
| Conditions | Yield |
|---|---|
|
With
graphitic carbon nitride; hydrazine hydrate;
In
water;
at 70 ℃;
for 24h;
chemoselective reaction;
Irradiation;
Sealed tube;
Green chemistry;
|
100% |
|
With
hydrogen;
In
water;
at 60 ℃;
for 3h;
under 760.051 Torr;
Green chemistry;
|
99% |
|
With
hydrogen;
In
tetrahydrofuran; water;
at 120 ℃;
for 6h;
under 22502.3 Torr;
Autoclave;
|
99% |
|
With
hydrogen;
In
water;
at 60 ℃;
for 4h;
under 760.051 Torr;
|
99% |
|
With
hydrazine hydrate;
In
ethanol;
at 40 ℃;
for 0.5h;
|
99% |
|
With
hydrogen; triethylamine;
In
ethanol; water;
at 110 ℃;
for 44h;
under 30003 Torr;
Autoclave;
|
99% |
|
With
hydrogen; triethylamine;
In
ethanol; water;
at 110 ℃;
for 44h;
under 30003 Torr;
Autoclave;
|
99% |
|
With
hydrogen;
In
tetrahydrofuran;
at 60 ℃;
under 15001.5 Torr;
Flow reactor;
|
99.4% |
|
With
hydrogen;
palladium on activated charcoal;
In
ethanol;
at 40 ℃;
for 2h;
|
98% |
|
With
hydrogen;
In
2-methyltetrahydrofuran; water;
at 40 ℃;
for 24h;
under 15001.5 Torr;
chemoselective reaction;
|
98% |
|
With
hydrazine hydrate;
In
ethanol;
at 20 ℃;
for 4h;
chemoselective reaction;
|
97% |
|
With
hydrazine hydrate;
pyrographite;
In
ethanol;
for 2h;
Heating;
|
96% |
|
With
titanium(III) chloride;
In
acetic acid;
at 19 ℃;
for 0.116667h;
|
95.5% |
|
With
sodium dithionite; potassium carbonate;
1,1′-dioctyl-4,4′-bipyridinium;
In
dichloromethane; water;
at 35 ℃;
for 3h;
|
95% |
|
With
hydrogen;
nickel;
In
ethanol;
at 20 ℃;
for 0.75h;
under 2327.17 Torr;
|
95% |
|
With
hydrazine;
Montmorillonite;
In
ethanol;
for 2h;
Heating;
|
94% |
|
With
tetrahydroxydiboron; 5%-palladium/activated carbon; water;
In
acetonitrile;
at 50 ℃;
for 24h;
|
94% |
|
With
tetrahydroxydiboron; palladium on activated charcoal; water;
In
acetonitrile;
at 50 ℃;
for 24h;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
94% |
|
With
formic acid; triethylamine;
for 24h;
chemoselective reaction;
|
93% |
|
With
hydrogen;
In
ethanol; ethyl acetate;
at 100 ℃;
under 6205.94 Torr;
Flow reactor;
|
93% |
|
With
tetrahydroxydiboron; copper diacetate;
In
acetonitrile;
at 80 ℃;
for 24h;
Temperature;
chemoselective reaction;
Catalytic behavior;
Schlenk technique;
|
92% |
|
With
C36H56Cl3CrN2O; magnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane;
In
tetrahydrofuran;
at 60 ℃;
for 24h;
chemoselective reaction;
Inert atmosphere;
|
92% |
|
With
triethylamine;
In
water;
at 80 ℃;
for 4h;
chemoselective reaction;
Inert atmosphere;
Green chemistry;
|
91% |
|
With
hydrogen;
In
water;
at 100 ℃;
under 7500.75 Torr;
chemoselective reaction;
Green chemistry;
|
91% |
|
With
iron; acetic acid;
|
90% |
|
With
C12H12ClN2ORu(1+); hydrazine hydrate;
In
ethanol; water;
at 80 ℃;
for 24h;
|
90% |
|
With
palladium 10% on activated carbon; ammonium formate; silica gel;
In
methanol;
for 1.5h;
Milling;
|
90% |
|
With
hydrogen;
In
tert-butyl alcohol;
at 120 ℃;
for 20h;
under 30003 Torr;
Autoclave;
|
90% |
|
With
pyrographite; hydrazine hydrate;
In
methanol;
at 80 ℃;
for 12h;
|
90% |
|
With
4,4,4',4',6,6,6',6'-octamethyl-2,2'-bi(1,3,2-dioxaborinane); potassium hydride;
In
ethanol;
at 60 ℃;
for 18h;
Schlenk technique;
Inert atmosphere;
Sealed tube;
|
90% |
|
With
hydrazine;
In
ethanol;
at 60 ℃;
for 12h;
chemoselective reaction;
Inert atmosphere;
Sealed tube;
|
88% |
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; water;
at 20 ℃;
for 2h;
chemoselective reaction;
Inert atmosphere;
Green chemistry;
|
88% |
|
With
sodium tetrahydroborate; iron; water;
at 20 ℃;
for 2h;
|
88% |
|
8-nitroquinoline;
With
iron; acetic acid;
at 65 ℃;
for 2h;
With
sodium hydrogencarbonate;
In
water; ethyl acetate;
|
83% |
|
With
ammonium sulfide;
In
ethanol;
for 2h;
Heating;
|
81% |
|
8-nitroquinoline;
In
methanol; water;
at 20 ℃;
With
sodium tetrahydroborate;
In
methanol; water;
at 50 ℃;
for 2h;
|
80% |
|
With
phenylsilane; C78H88Cl2N4Ni;
In
tetrahydrofuran;
at 60 ℃;
for 1h;
chemoselective reaction;
Inert atmosphere;
|
78% |
|
With
sodiumsulfide nonahydrate; acetic acid;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 20h;
Reagent/catalyst;
Temperature;
chemoselective reaction;
Green chemistry;
|
78% |
|
With
tris(bipyridine)ruthenium(II) dichloride hexahydrate; ascorbic acid;
In
methanol; water;
at 20 ℃;
for 4h;
chemoselective reaction;
Schlenk technique;
Inert atmosphere;
Irradiation;
Green chemistry;
|
74.5% |
|
8-nitroquinoline;
With
sodium sulfide; ammonium chloride;
In
methanol;
for 2h;
Reflux;
With
hydrogenchloride;
In
methanol; water;
|
72% |
|
With
nickel(II) nitrate hexahydrate; hydrazine hydrate; sodium hydroxide;
In
water; isopropyl alcohol;
Reflux;
|
72% |
|
With
nickel(II) nitrate hexahydrate; hydrazine hydrate; sodium hydroxide;
In
water; isopropyl alcohol;
Reflux;
|
72% |
|
With
hydrogenchloride; tin(II) chloride dihdyrate;
|
69% |
|
With
hydrogenchloride; tin(ll) chloride;
In
methanol;
at 20 ℃;
for 5.5h;
Reflux;
|
57% |
|
With
sodium tetrahydroborate; iron(III) trifluoromethanesulfonate; ethanol;
for 18h;
Inert atmosphere;
Green chemistry;
|
51% |
|
With
hydrogenchloride; tin;
|
|
|
With
hydrogenchloride; tin(ll) chloride;
|
|
|
With
hydrogenchloride; iron;
|
|
|
With
iron; acetic acid;
|
|
|
With
ethanol; iron; calcium chloride;
|
|
|
With
ethanol; nickel;
Hydrogenation;
|
|
|
With
ethanol; ethyl acetate; platinum;
Hydrogenation.und Aether;
|
|
|
With
palladium on activated charcoal; acetic acid;
Hydrogenation;
|
|
|
With
hydrazine hydrochloride; ammonia;
at 100 ℃;
|
|
|
With
ammonia; sulfur;
at 100 ℃;
|
|
|
With
palladium on activated charcoal; ethanol; hydrazine hydrate;
|
|
|
With
ethanol; nickel; hydrazine hydrate;
|
|
|
With
sodium sulfide; ethanol; ammonium chloride;
|
|
|
With
phenylsilane; triphenylphosphine; iron(II) bromide;
In
toluene;
at 110 ℃;
for 16h;
Inert atmosphere;
|
61 %Chromat. |
|
With
hydrazine hydrate;
In
tetrahydrofuran;
at 100 ℃;
for 10h;
chemoselective reaction;
|
98 %Chromat. |
|
With
formic acid; [Mo3S4H3(dmpe)3](BPh4); triethylamine;
In
tetrahydrofuran;
at 70 ℃;
for 24h;
chemoselective reaction;
Inert atmosphere;
|
> 99 %Chromat. |
|
With
hydrogenchloride; tin(II) chloride dihdyrate;
In
ethanol; water;
at 20 - 50 ℃;
for 2.5h;
|
|
|
With
iron(II) fluoro{tris[2-(diphenylphosphino)phenyl]phospino}tetrafluoroborate; hydrogen; trifluoroacetic acid;
In
tert-Amyl alcohol;
at 120 ℃;
for 2h;
under 15001.5 Torr;
Catalytic behavior;
Inert atmosphere;
Autoclave;
|
78 %Chromat. |
|
With
cobalt; hydrazine;
In
water;
at 20 ℃;
for 4h;
Reagent/catalyst;
Time;
chemoselective reaction;
|
|
|
With
carbon monoxide; water;
In
tetrahydrofuran;
at 125 ℃;
for 24h;
under 22502.3 - 45004.5 Torr;
Inert atmosphere;
Autoclave;
|
93 %Chromat. |
|
With
formic acid; triethylamine;
In
tetrahydrofuran;
at 100 ℃;
for 15h;
chemoselective reaction;
Inert atmosphere;
Sealed tube;
|
92 %Chromat. |
|
With
hydrogen;
In
ethanol;
at 110 ℃;
for 15h;
under 22502.3 Torr;
chemoselective reaction;
Autoclave;
|
> 99 %Chromat. |
|
With
hydrogen;
In
neat (no solvent);
at 95 ℃;
for 11h;
under 7500.75 Torr;
Catalytic behavior;
Green chemistry;
|
|
|
With
hydrogen;
In
toluene;
at 140 ℃;
for 15h;
under 7500.75 Torr;
chemoselective reaction;
Autoclave;
|
|
|
With
hydrogen; triethylamine;
In
ethanol; water;
at 70 ℃;
for 20h;
under 15001.5 Torr;
Reagent/catalyst;
Autoclave;
|
|
|
With
hydrogen;
In
tetrahydrofuran; water;
at 90 ℃;
for 3h;
under 37503.8 Torr;
Autoclave;
|
|
|
With
formic acid; triethylamine;
In
N,N-dimethyl-formamide;
at 130 ℃;
for 2h;
chemoselective reaction;
|
|
|
With
[Mo3S4Cl3(4,4'-dinonyl-2,2'-bipyridine)3](PF6); hydrogen;
In
methanol;
at 70 ℃;
for 18h;
under 15001.5 Torr;
Autoclave;
|
> 99 %Chromat. |
|
With
hydrogen;
In
neat (no solvent);
at 105 ℃;
for 6h;
under 15001.5 Torr;
chemoselective reaction;
|
|
|
With
hydrogen; hydrazine hydrate;
In
ethanol;
at 40 ℃;
for 2h;
under 15001.5 Torr;
chemoselective reaction;
Catalytic behavior;
Autoclave;
|
|
|
With
hydrogen;
In
methanol;
at 110 ℃;
for 13h;
under 22502.3 Torr;
chemoselective reaction;
Autoclave;
|
> 99 %Chromat. |
|
With
hydrogen;
In
water;
at 100 ℃;
for 11h;
under 37503.8 Torr;
chemoselective reaction;
Autoclave;
Green chemistry;
|
|
|
With
hydrogen;
In
water;
at 25 ℃;
for 2.5h;
under 3620.13 Torr;
|
89 %Spectr. |
|
With
hydrogen;
In
tetrahydrofuran; water;
at 60 ℃;
for 6h;
under 37503.8 Torr;
Time;
Catalytic behavior;
Autoclave;
|
|
|
With
hydrazine hydrate;
In
ethanol;
at 30 ℃;
for 1h;
chemoselective reaction;
Autoclave;
|
|
|
With
hydrogen;
In
methanol;
at 110 ℃;
for 5h;
under 22502.3 Torr;
Autoclave;
|
|
|
With
hydrogen;
In
methanol;
at 60 ℃;
for 3h;
under 22502.3 Torr;
Temperature;
Time;
Autoclave;
Green chemistry;
|
|
|
With
hydrogen;
In
ethanol;
at 50 ℃;
for 2h;
under 7500.75 Torr;
Autoclave;
|
|
|
With
sodium tetrahydroborate; C18H17ClN4PdS2;
In
ethanol;
at 24.84 ℃;
for 2h;
|
90 %Chromat. |
|
With
borane-ammonia complex;
In
methanol; water;
at 30 ℃;
for 0.0833333h;
|
>99 %Chromat. |
|
With
hydrogen;
In
ethanol; water;
at 20 ℃;
for 5h;
|
|
|
With
iron(III) chloride; pyrographite; hydrazine hydrate;
In
methanol;
at 80 ℃;
for 12h;
|
|
|
With
hydrogen;
In
water; glycerol;
at 20 ℃;
for 7h;
under 760.051 Torr;
chemoselective reaction;
Schlenk technique;
Green chemistry;
|
80 %Chromat. |
|
With
hydrogen;
In
ethanol;
at 50 ℃;
for 1h;
under 3750.38 Torr;
chemoselective reaction;
Autoclave;
|
8-quinolinol

8-nitroquinoline
8-nitro-quinoline N-oxide
4-chloro-8-nitroquinoline
4-phenyl-1,10-phenanthroline
Ν-(quinolinyl)-o-aminobenzoic acid
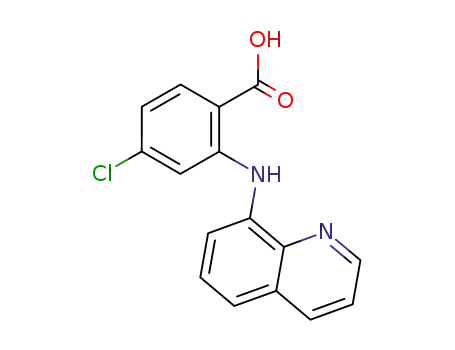
2-[8]quinolylamino-4-chloro-benzoic acid
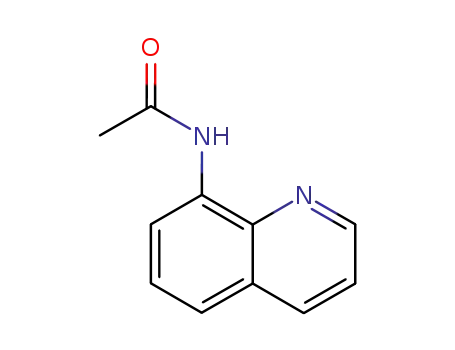
N-(quinolin-8-yl)acetamide