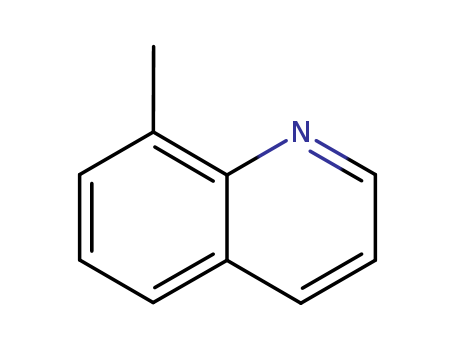

CasNo: 611-32-5
MF: C10H9N
Appearance: clear yellow liquid
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 45, p. 1514, 1980 DOI: 10.1021/jo01296a035 |
|
Air & Water Reactions |
Slightly soluble in water. |
|
Reactivity Profile |
8-Methylquinoline may be sensitive to exposure to light. May react vigorously with strong oxidizing agents and strong acids . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Fire Hazard |
8-Methylquinoline is combustible. |
|
Purification Methods |
Purify it as for 2-methylquinoline. The phosphate and picrate have m 158o and m 201o, respectively. [Beilstein 20 III/IV 3500, 20/7 V 405.] |
|
Definition |
ChEBI: A methylquinoline carrying a methyl substituent at position 8. |
|
General Description |
Yellow liquid or oil. |
InChI:InChI=1/C10H9N/c1-8-4-2-5-9-6-3-7-11-10(8)9/h2-7H,1H3
-
We report the design of a bifunctional m...
Photoactive two-dimensional covalent org...
Herein, an iron(II)-catalyzed biomimetic...
N-heterocycles are key structures for ma...
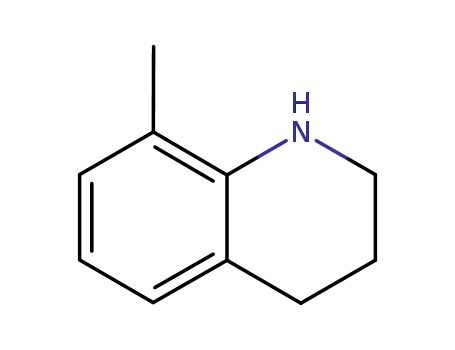
8-methyl-1,2,3,4-tetrahydroquinoline

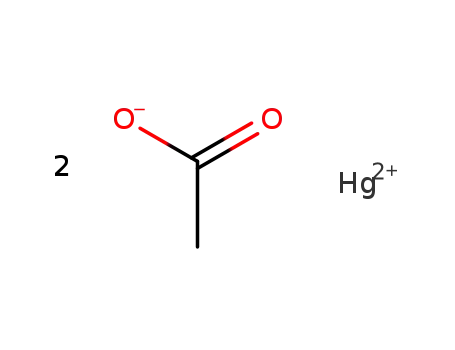
mercury(II) diacetate

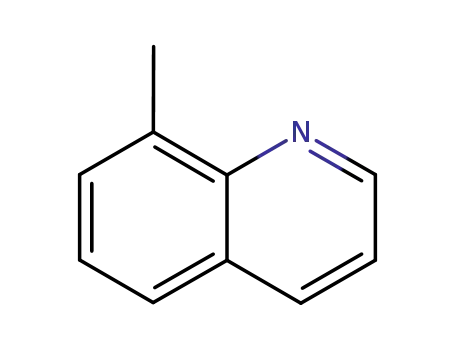
8-methylquinoline
| Conditions | Yield |
|---|---|
|
|

8-methyl-1,2,3,4-tetrahydroquinoline


8-methylquinoline
| Conditions | Yield |
|---|---|
|
With
diethylazodicarboxylate;
In
chloroform; toluene;
at 20 ℃;
for 12h;
|
93% |
|
With
[(CH3)2NH2]4[(UO2)4(Co-TCPP)3]·6DMF·33H2O; oxygen;
In
N,N-dimethyl-formamide;
at 119.84 ℃;
for 9h;
|
93% |
|
With
6C44H32N6O4Ru(2+)*12Hf(2+)*8O(2-)*14HO(1-)*6C16H22ClCoN5O6(1-);
In
2,2,2-trifluoroethanol; acetonitrile;
at 20 ℃;
for 24h;
Catalytic behavior;
Inert atmosphere;
Irradiation;
|
91% |
|
With
iron oxide surrounded by nitrogen doped graphene shell immobilized on carbon support;
In
n-heptane;
at 100 ℃;
for 12h;
under 11251.1 Torr;
Autoclave;
|
88% |
|
With
[Ru(1,10-phenanthroline-5,6-dione)3](PF6)2; oxygen; tetra-(n-butyl)ammonium iodide;
In
acetonitrile;
at 27 ℃;
for 72h;
under 760.051 Torr;
|
86% |
|
With
oxygen;
In
N,N-dimethyl acetamide;
at 20 ℃;
Schlenk technique;
Irradiation;
|
84% |
|
With
C22H29IrN4O4S;
In
water;
for 30h;
Reflux;
Schlenk technique;
|
83% |
|
With
tris(bipyridine)ruthenium(II) dichloride hexahydrate; chloropyridinecobaloxime(III);
In
ethanol;
at 30 ℃;
for 6h;
Schlenk technique;
Inert atmosphere;
Irradiation;
|
81% |
|
With
manganese(II) phthalocyanin; 2-(4-nitrophenyl)hydrazin-1-carboxylic acid ethyl ester; oxygen;
In
acetonitrile;
at 70 ℃;
for 15h;
|
80% |
|
With
trimethylamine-N-oxide; Co(salophen)-HQ;
In
dimethyl sulfoxide;
at 90 ℃;
for 36h;
Schlenk technique;
Green chemistry;
|
80% |
|
With
[iPrPN(H)P]2Fe(H)(CO)(BH4);
In
5,5-dimethyl-1,3-cyclohexadiene;
at 140 ℃;
for 30h;
Inert atmosphere;
Schlenk technique;
Glovebox;
|
78% |
|
With
rose bengal; oxygen;
In
N,N-dimethyl acetamide;
at 20 ℃;
for 24h;
Irradiation;
|
78% |
|
With
rose bengal;
In
N,N-dimethyl acetamide;
at 20 ℃;
for 24h;
Irradiation;
|
78% |
|
With
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrabutylammonium tetrafluoroborate;
In
water; acetonitrile;
at 20 ℃;
for 4h;
Electrochemical reaction;
|
77% |
|
With
iodine; oxygen;
In
1,2-dichloro-benzene; toluene;
at 160 ℃;
for 30h;
|
74% |
|
With
mesoporous Co3O4; air;
In
dimethyl sulfoxide;
at 140 ℃;
for 24h;
under 760.051 Torr;
Schlenk technique;
|
73% |
|
With
oxygen; potassium carbonate;
In
methanol;
at 60 ℃;
for 9h;
under 760.051 Torr;
|
72% |
|
With
C55H49N4OP2Ru;
In
o-xylene;
at 140 ℃;
for 48h;
under 750.075 Torr;
Inert atmosphere;
Schlenk technique;
Green chemistry;
|
72% |
|
With
oxygen; 1-(2,2-diphenyl-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one;
In
1-methyl-pyrrolidin-2-one;
at 20 ℃;
for 5h;
Irradiation;
Green chemistry;
|
71% |
|
With
oxygen; iron(II) chloride;
In
para-xylene; dimethyl sulfoxide;
at 110 ℃;
for 24h;
Schlenk technique;
|
70% |
|
With
tris(pentafluorophenyl)borate;
In
para-xylene;
at 150 ℃;
for 22h;
Inert atmosphere;
Sealed tube;
|
70% |
|
With
cobalt(II) 5,10,15,20-tetraphenylporphyrin; oxygen;
In
N,N-dimethyl-formamide;
for 13h;
|
67% |
|
With
copper(I) oxide; dmap; N-hydroxyphthalimide; oxygen;
In
acetonitrile;
at 120 ℃;
for 12h;
Sealed tube;
|
66% |
|
With
copper(I) oxide; dmap; N-hydroxyphthalimide; oxygen;
In
acetonitrile;
at 120 ℃;
for 12h;
|
66% |
|
With
tris(bipyridine)ruthenium(II) dichloride hexahydrate;
In
acetonitrile;
at 25 ℃;
for 4h;
Temperature;
Irradiation;
Green chemistry;
|
65% |
|
With
potassium tert-butylate;
In
o-xylene;
at 140 ℃;
for 36h;
Inert atmosphere;
|
64% |
|
With
mercury(II) diacetate;
|
|
|
With
platinum; oxygen;
In
methanol;
at 40 ℃;
under 750.075 Torr;
Schlenk technique;
Sealed tube;
|
99.8 %Chromat. |
|
With
oxygen;
In
1,3,5-trimethyl-benzene;
at 80 ℃;
for 6.5h;
under 760.051 Torr;
|
|
|
With
tert.-butylhydroperoxide;
In
water;
at 20 ℃;
for 18h;
Sealed tube;
|
92 %Spectr. |
|
With
Pd-Ni bimetallic nanoparticles on MIL-100(Fe);
In
water;
at 130 ℃;
for 12h;
Sealed tube;
Inert atmosphere;
|
87 %Chromat. |
|
With
cobalt nanocrystals stabilized by nitrogen-doped graphitized carbon; air;
In
methanol;
at 50 ℃;
for 12h;
|
99.9 %Chromat. |

8-methyl-1,2,3,4-tetrahydroquinoline

mercury(II) diacetate
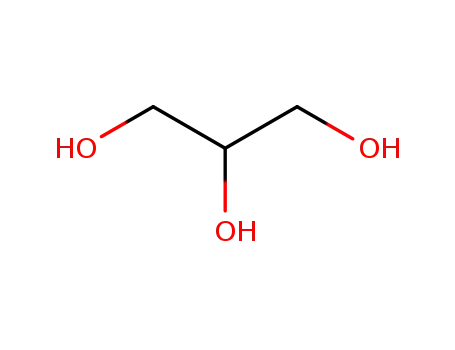
glycerol
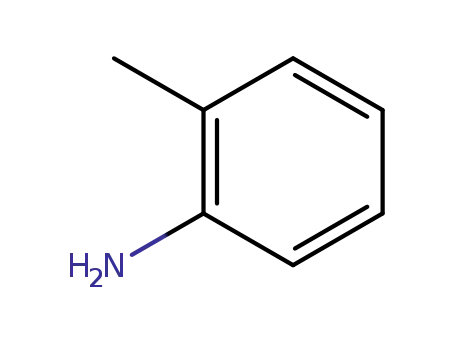
o-toluidine
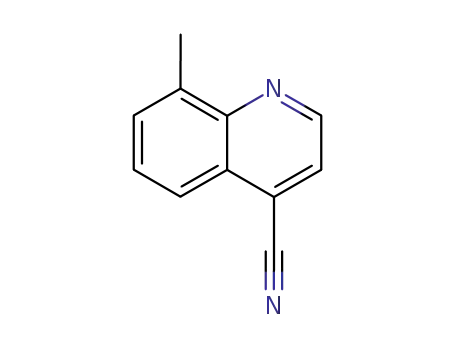
4-cyano-8-methylquinoline
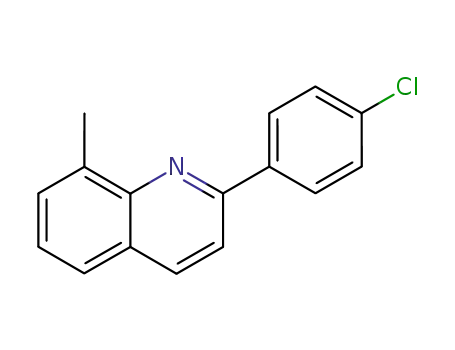
2-(4-chloro-phenyl)-8-methyl-quinoline
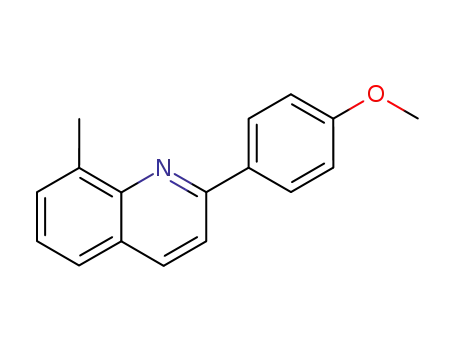
(2-(4-methoxyphenyl)-8-methylquinoline)
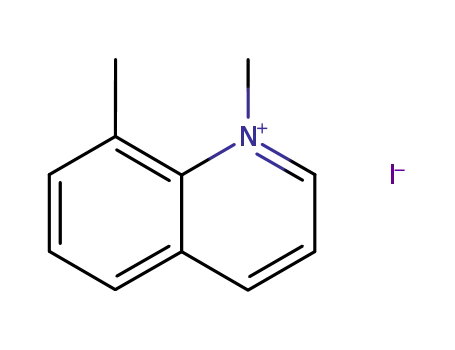
1,8-dimethylquinolin-1-ium iodide