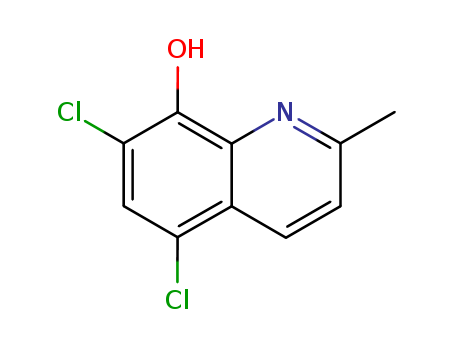

CasNo: 72-80-0
MF: C10H7Cl2NO
Appearance: yellowish to beige-brown powder
|
Manufacturing Process |
11.1 parts of 8-hydroxy-quinaldine are dissolved in 140 parts of formic acid. Chlorine is introduced into this solution under cooling, until the increase in weight corresponds to the required quantity of chlorine and a test of the chlorination mixtures gives no more dyestuff formation with diazo-benzene in an acetic acid solutionWhen the chlorination is complete, the reaction mixture is poured into 1,000 parts of water and treated with a dilute sodium bisulfite solution, until no more reaction may be observed with starch potassium iodide paper. Thereby the 5,7-dichloro-8-hydroxy-quinaldine separates out in form of a weakly yellowish colored precipitate. The same is filtered off and thoroughly washed with water.After drying, 15 parts of 5,7-dichloro-8-hydroxy-quinaldine melting at 111°C to 112°C are obtained. When recrystallized from alcohol, the product is obtained in voluminous, slightly yellowish needles having the melting point of 111.5°C to 112°C. |
|
Therapeutic Function |
Antibacterial |
|
Purification Methods |
Crystallise it from EtOH. [Beilstein 21/3 V 346.] |
|
Definition |
ChEBI: A monohydroxyquinoline that is quinolin-8-ol which is substituted by a methyl group at position 2 and by chlorine at positions 5 and 7. An antifungal and antibacterial, it was formerly used for topical treatment of skin conditions and vaginal infections. |
InChI:InChI=1/C10H7Cl2NO/c1-5-2-3-6-7(11)4-8(12)10(14)9(6)13-5/h2-4,14H,1H3
The invention provides a preparation met...
The invention provides a preparation met...
The invention belongs to the technical f...
The rare earth complex constructed based...
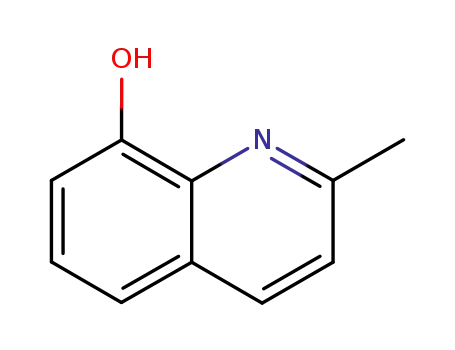
2-methyl-8-quinolinol

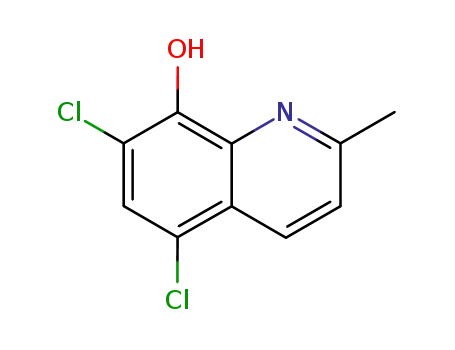
chloroquinaldol
| Conditions | Yield |
|---|---|
|
With
aluminum (III) chloride; N-chloro-succinimide;
In
dichloromethane;
at 35 ℃;
for 8h;
Temperature;
Solvent;
Darkness;
Green chemistry;
|
98.8% |
|
With
hydrogenchloride; chlorine;
In
water;
Reagent/catalyst;
Inert atmosphere;
Darkness;
|
98.9% |
|
With
hydrogenchloride; sodium hypochlorite;
In
water;
at 20 - 30 ℃;
for 4.5h;
Temperature;
|
97.7% |
|
With
formic acid; chlorine;
|
|
|
With
chlorine;
|
|
|
With
1,3-Dichloro-2-propanol; acetic acid;
for 3h;
Cooling with ice;
|
|
|
With
1,3-Dichloro-2-propanol; acetic acid;
for 3h;
Cooling with ice;
|
|
|
With
1,3-dichloro-5,5-dimethylhydantoin; acetic acid;
for 3h;
Cooling with ice;
|

2-methyl-8-quinolinol

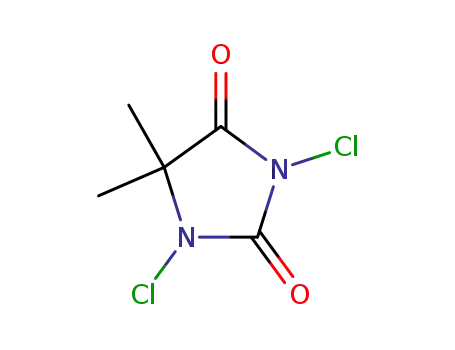
1,3-dichloro-5,5-dimethylhydantoin


chloroquinaldol
| Conditions | Yield |
|---|---|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 32 - 41 ℃;
for 10h;
Time;
Solvent;
Reagent/catalyst;
|
68.98% |

2-methyl-8-quinolinol
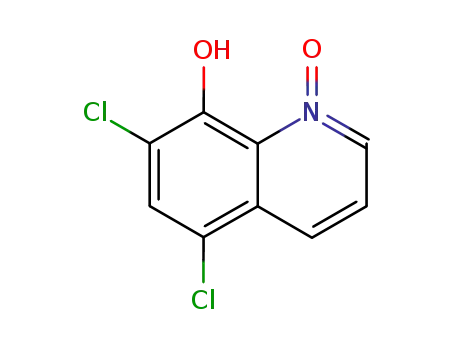
5,7-dichloro-8-hydroxyquinoline 1-oxide
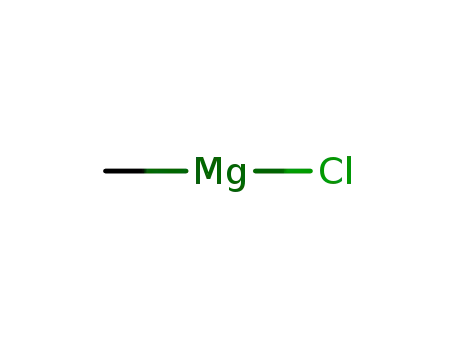
methylmagnesium chloride
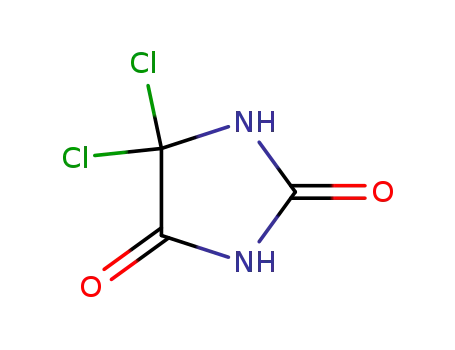
dichlorohydantoin
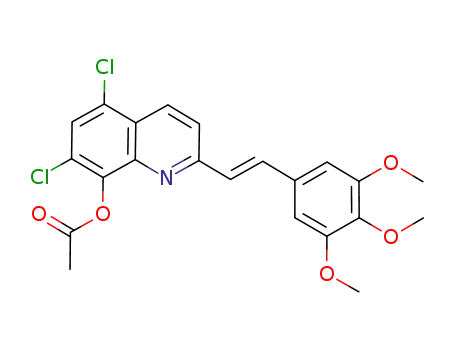
8-acetoxy-5,7-dichloro-2-[2-(3,4,5-trimethoxyphenyl)vinyl]quinoline
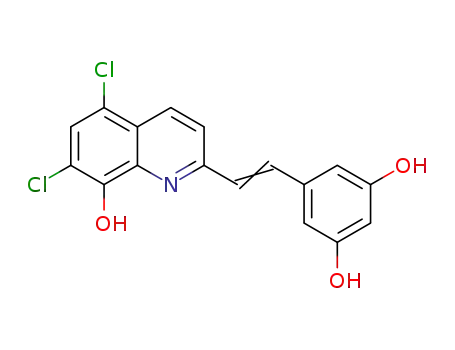
5,7-dichloro-2-[2-(3,5-dihydroxyphenyl)vinyl]quinolin-8-ol
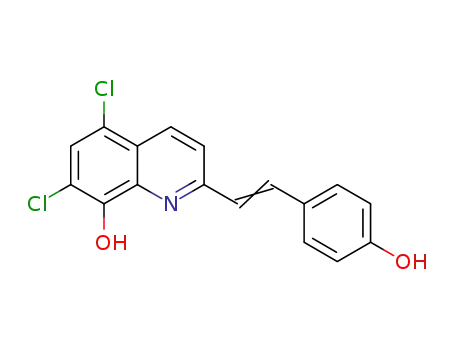
5,7-dichloro-2-[2-(4-hydroxyphenyl)vinyl]quinolin-8-ol
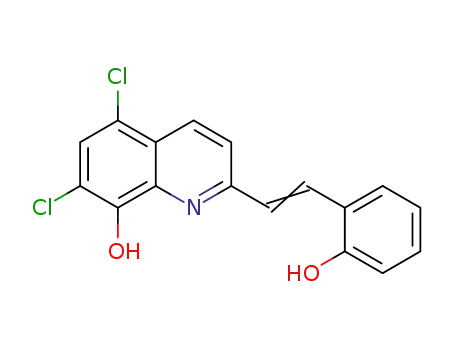
5,7-dichloro-2-[2-(2-hydroxyphenyl)vinyl]quinolin-8-ol