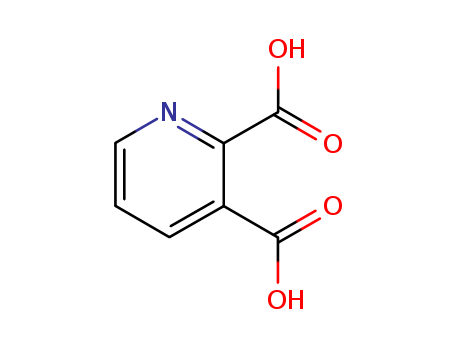

CasNo: 89-00-9
MF: C7H5NO4
Appearance: Colorless columnar crystal
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 71, p. 3020, 1949 DOI: 10.1021/ja01177a021 |
|
Hazard |
A poison by skin contact. Moderately toxic by ingestion. A mild skin irritant. |
|
Biological Activity |
Endogenous NMDA agonist and transmitter candidate. May distinguish between NMDA receptor subtypes. |
|
Safety Profile |
A poison by skin contact. Moderately toxic by ingestion. Experimental reproductive effects. A mdd skinn irritant. When heated to decomposition it emits toxic vapors of NOx. |
|
Industrial uses |
The use of quinolinic acid during flotation of hematite results in the adsorption of quinoline on hematite, allowing amine to selectively adsorb onto the hematite surface. |
InChI:InChI=1/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12)/p-2
The biosynthesis of quinolinate 3, the p...
The invention discloses a preparation me...
The synthesis of quinolinic acid from tr...
The invention relates to compound prepar...
The invention discloses a 2, 3 - pyridin...
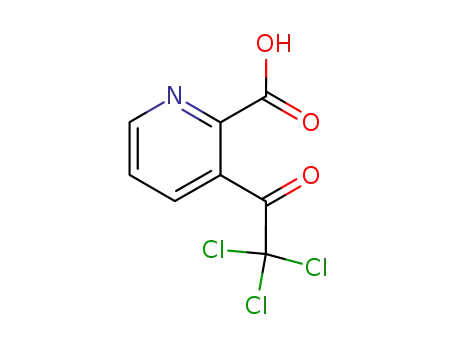
3-trichloroacetyl-pyridine-2-carboxylic acid

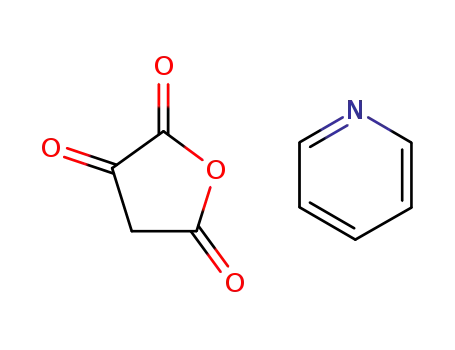
furan-2,3,5(4H)-trione pyridine (1:1)

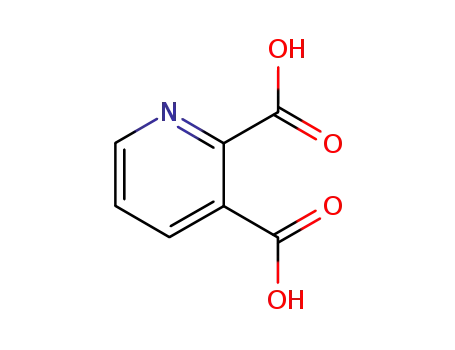
Pyridine-2,3-dicarboxylic acid

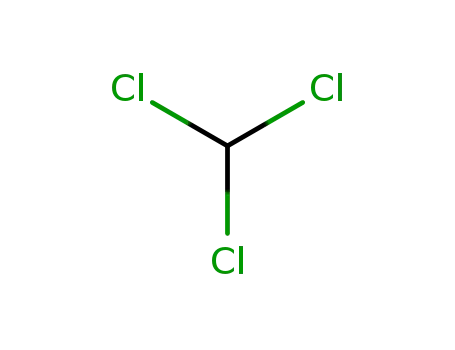
chloroform
| Conditions | Yield |
|---|---|
|
|
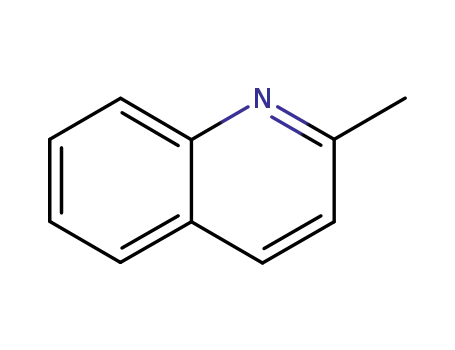
2-methylquinoline

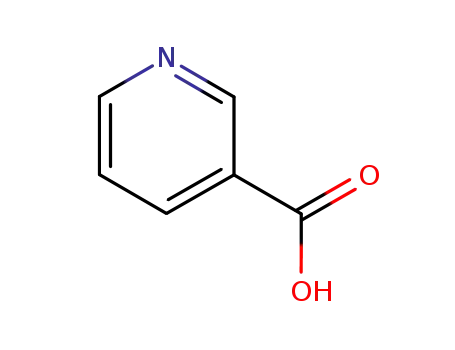
nicotinic acid

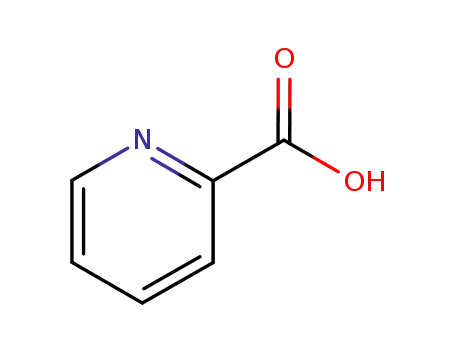
2-Picolinic acid

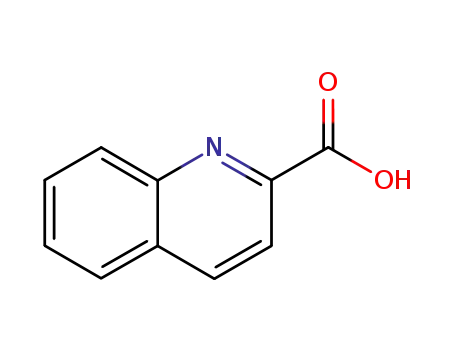
quinoline-2-carboxylic acid


Pyridine-2,3-dicarboxylic acid

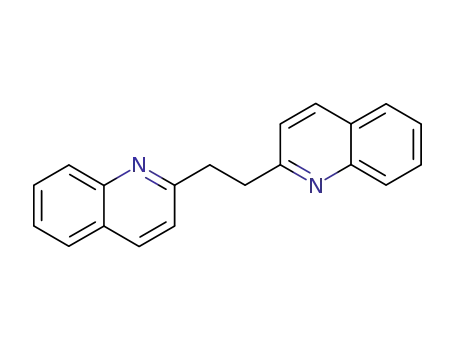
1,2-Bis(quinolin-2-yl)ethane

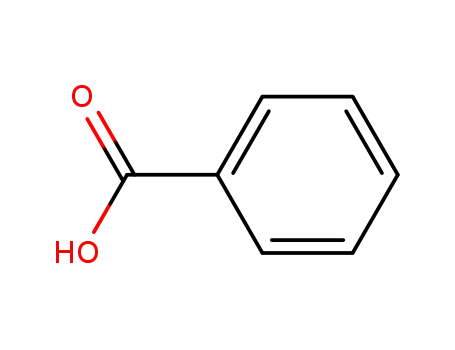
benzoic acid
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide; oxygen;
In
water;
at 200 ℃;
for 1h;
under 58840.6 Torr;
Product distribution;
var. time, var. temperatures;
|
41.7% 2.2% 8.1% 41.3% 0.7% |
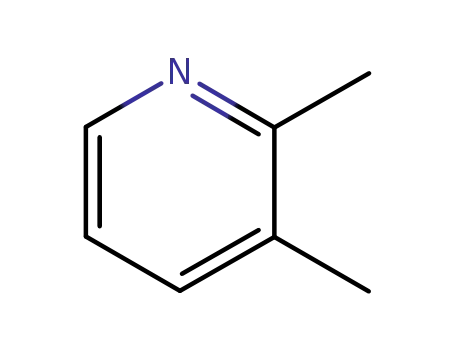
2,3-Lutidine
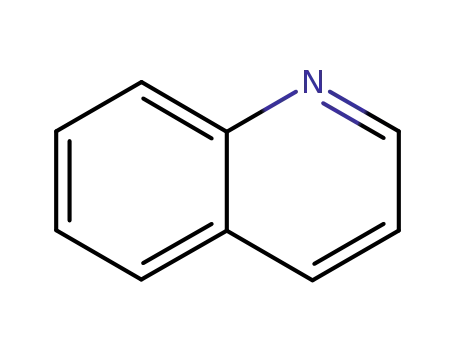
quinoline
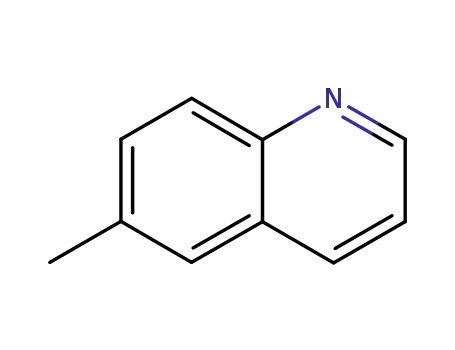
6-methylquinoline
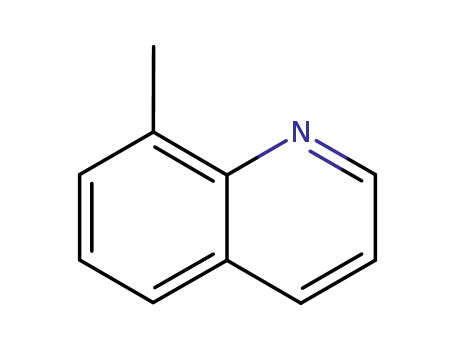
8-methylquinoline
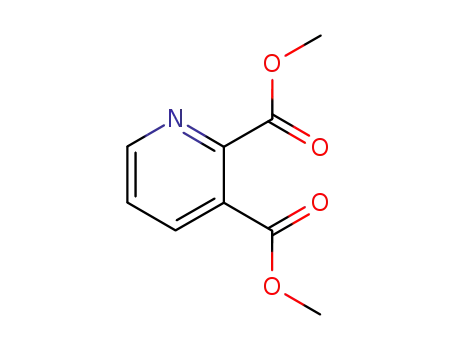
dimethyl 2,3-pyridinedicarboxylate
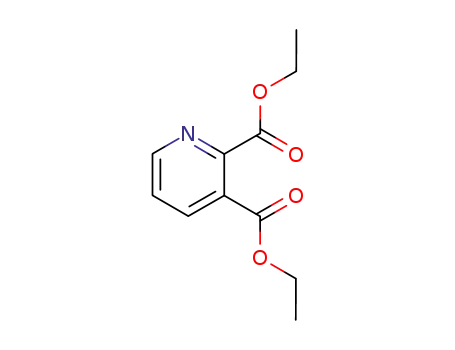
2,3-diethyl pyridinedicarboxylate
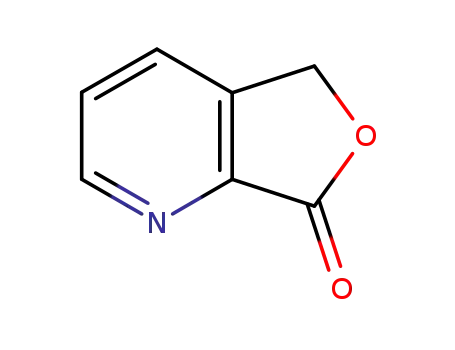
furo[3,4-b]pyridine-7(5H)-one
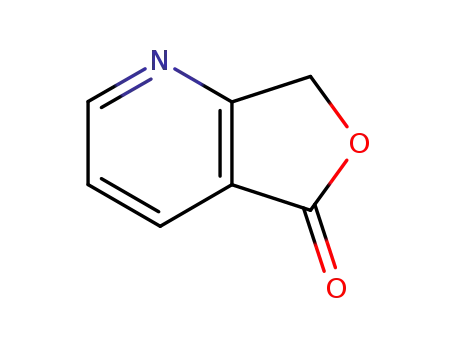
7H-furo[3,4-b]pyridin-5-one