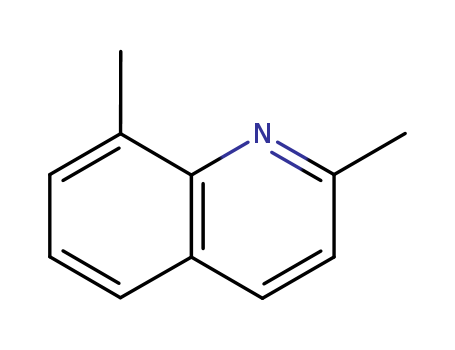

CasNo: 1463-17-8
MF: C11H11N
Appearance: liquid
InChI:InChI=1/C11H11N/c1-8-4-3-5-10-7-6-9(2)12-11(8)10/h3-7H,1-2H3
A heterogeneous system Fe(CrO2)2–TiO2/X ...
A general method for the oxidative subst...
[Figure not available: see fulltext.] A ...
One-pot tandem synthesis was developed f...
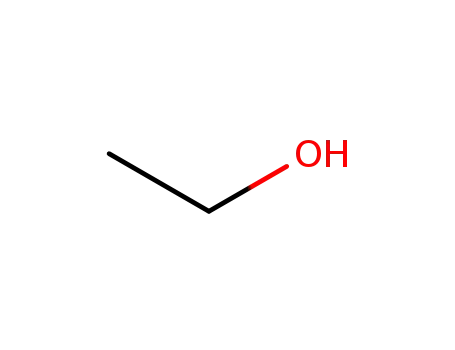
ethanol

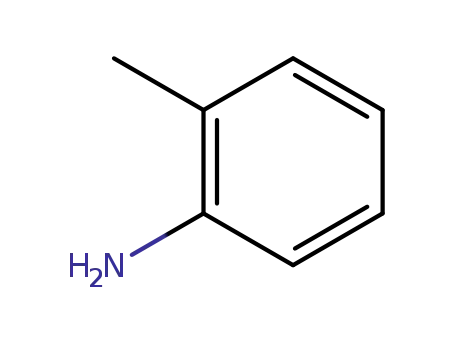
o-toluidine

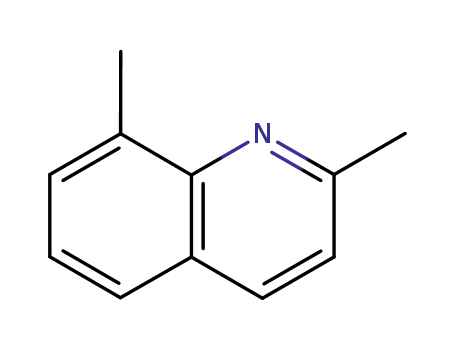
2,8-dimethylquinoline
| Conditions | Yield |
|---|---|
|
ethanol;
With
iron chromite;
for 24h;
With
dihydrogen peroxide;
In
water;
at 20 ℃;
for 2h;
Irradiation;
o-toluidine;
In
water;
at 20 ℃;
for 0.0833333h;
|
94% |
|
ethanol;
With
iron chromite; dihydrogen peroxide;
In
water;
at 20 ℃;
for 2h;
UV-irradiation;
o-toluidine;
In
water;
for 0.0833333h;
|
94% |
|
With
iron(III) chloride hexahydrate;
In
tetrachloromethane;
at 140 ℃;
for 4h;
Autoclave;
Inert atmosphere;
Sealed tube;
|
85% |
|
With
copper(II) oxide;
In
water;
at 25 ℃;
UV-irradiation;
|
85% |
|
With
platinum-loaded TiO2 nanoparticles;
at 30 ℃;
for 4h;
Irradiation;
Inert atmosphere;
|
72% |
|
With
2,4,6-trimethyl-pyridine; oxygen; palladium diacetate; trifluoroacetic acid;
at 150 ℃;
for 16h;
Schlenk technique;
|
72% |
|
With
transition metal catalyst; pyridine derviative; acid derviative;
at 150 ℃;
for 16h;
Green chemistry;
|
72% |
|
With
gold on titanium oxide;
at 30 ℃;
for 4h;
UV-irradiation;
Inert atmosphere;
|
68% |
|
at 30 ℃;
for 4h;
UV-irradiation;
Inert atmosphere;
|
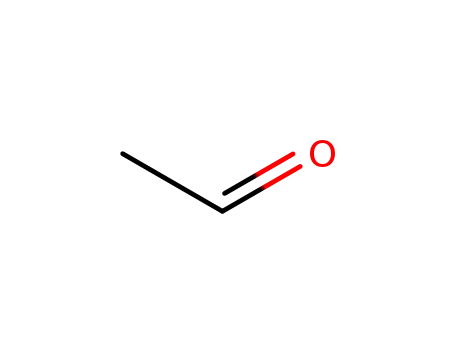
acetaldehyde


o-toluidine


2,8-dimethylquinoline
| Conditions | Yield |
|---|---|
|
bis(1,5-cyclooctadiene)diiridium(I) dichloride;
In
dimethyl sulfoxide;
at 90 ℃;
for 17h;
|
74% |
|
With
di-μ-chlorobis(norbornadiene)dirhodium(I); nitrobenzene;
In
ethanol;
at 180 ℃;
for 4h;
|
24% |
|
With
hydrogenchloride; zinc(II) chloride;
|
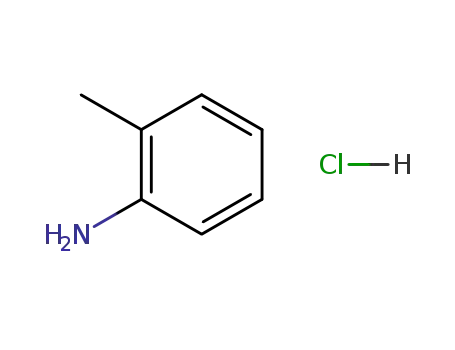
o-toluidine hydrochloride

acetaldehyde

o-toluidine
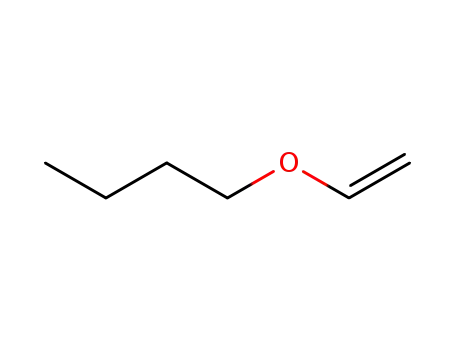
-butyl vinyl ether
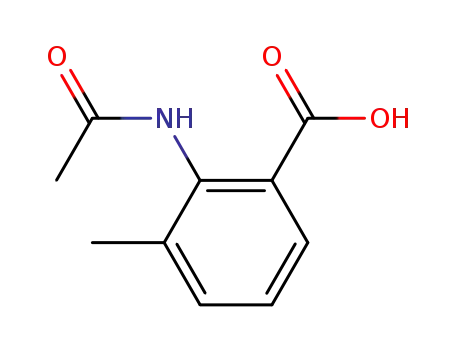
2-acetylamino-3-methylbenzoic acid
2,2'-(2-phenylpropane-1,3-diyl)bis(8-methylquinoline)