

CasNo: 158966-92-8
MF: C35H36ClNO3S
|
Therapeutic Function |
Anti-asthmatic |
|
Mechanism of action |
Montelukast was developed from other weakly antagonistic quinoline derivatives. A number of changes can be made to the structure without the loss of activity. These include changing the double bond between the two aromatic rings to an ether linkage, reducing the quinoline ring, changing the chlorine to a fluorine, and/or exchanging the sulfur for an amide group. |
|
Pharmacokinetics |
Montelukast is a high-affinity, selective antagonist of the cysLT1 receptor. It is rapidly absorbed orally, with a bioavailability of 64%. Montelukast is 99% bound to plasma proteins and is extensively metabolized in the liver by CYP3A4 and CYP2C9 to oxidated products. CYP3A4 oxidizes the sulfur and the C-21 benzylic carbon, whereas CYP2C9 is selectively responsible for the methyl hydroxylation. |
|
Side effects |
Montelukast did not demonstrate any significant adverse effects greater than placebo in clinical trials; however, because it is metabolized by the cytochrome P450 (CYP450) enzymes, its plasma levels should be monitored when coadministered with CYP450-inducing drugs, such as phenobarbital, rifampin, and phenytoin. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Extensively metabolised in the liver by cytochrome P450 isoenzymes CYP3A4, CYP2A6, and CYP2C9. Excreted principally in the faeces via the bile. Metabolites have minimal therapeutic activity. |
InChI:InChI=1/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/t32-/m1/s1
The invention provides a novel monteluka...
The invention relates to a series of nov...
PROBLEM TO BE SOLVED: To provide a metho...
PROBLEM TO BE SOLVED: To provide a metho...
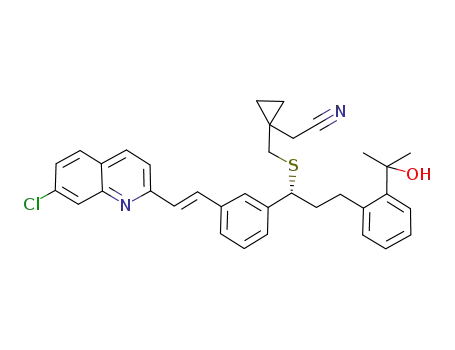
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile

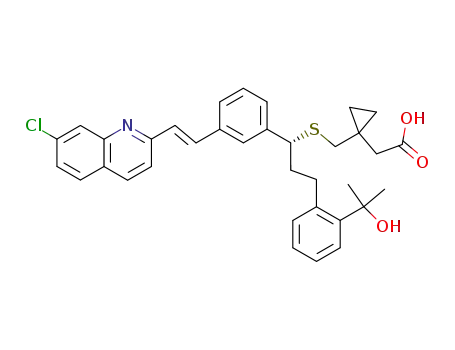
montelukast
| Conditions | Yield |
|---|---|
|
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile;
With
sodium hydroxide; ethanol; water;
for 15h;
Heating / reflux;
With
acetic acid;
In
ethanol; water; toluene;
Product distribution / selectivity;
|
91% |
|
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile;
With
sodium hydroxide; ethanol; water;
for 30h;
Heating / reflux;
With
water; acetic acid;
In
toluene;
for 0.5h;
pH=5.6;
Product distribution / selectivity;
|
86% |
|
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile;
With
sodium hydroxide; water;
tetrabutylammomium bromide;
In
toluene;
at 120 ℃;
for 168h;
With
acetic acid;
In
water; toluene;
Product distribution / selectivity;
|
83% |
|
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile;
With
sodium hydroxide; ethanol; water;
for 25h;
Heating / reflux;
With
acetic acid;
In
water; toluene;
for 0.5h;
pH=5 - 5.5;
Product distribution / selectivity;
|
|
|
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl)acetonitrile;
With
sodium hydroxide; water;
at 111 - 130 ℃;
for 20h;
With
water; acetic acid;
at 90 ℃;
for 1h;
pH=~ 11;
Product distribution / selectivity;
|
|
|
With
caustic lye;
at 120 ℃;
for 12h;
|
1.32 g |
![(1-{1-(R)-(3-bromophenyl)-3-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-propylsulfanylmethyl}-cyclopropyl)-acetic acid](/upload/2025/3/f842b17b-a67e-4ff7-999a-e67d99853213.png)
(1-{1-(R)-(3-bromophenyl)-3-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-propylsulfanylmethyl}-cyclopropyl)-acetic acid

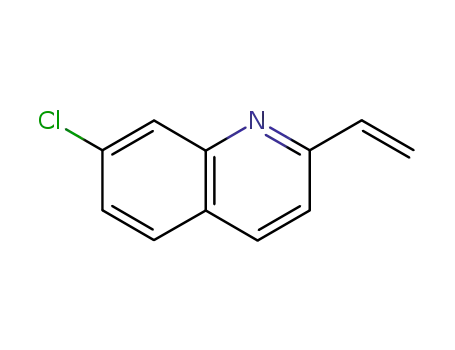
7-chloro-2-ethenylquinoline


montelukast
| Conditions | Yield |
|---|---|
|
With
triethylamine;
palladium diacetate; tris-(o-tolyl)phosphine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 4.25h;
|
85% |
|
With
triethylamine;
palladium diacetate; tris-(o-tolyl)phosphine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 4h;
Product distribution / selectivity;
|
67.6% |
|
(1-{1-(R)-(3-bromophenyl)-3-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-propylsulfanylmethyl}-cyclopropyl)-acetic acid; 7-chloro-2-ethenylquinoline;
With
triethylamine;
palladium diacetate; tris-(o-tolyl)phosphine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 4h;
With
water; citric acid;
In
ethyl acetate; N,N-dimethyl-formamide; toluene;
pH=3 - 4;
|
|
|
(1-{1-(R)-(3-bromophenyl)-3-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-propylsulfanylmethyl}-cyclopropyl)-acetic acid; 7-chloro-2-ethenylquinoline;
palladium diacetate; tris-(o-tolyl)phosphine;
In
N,N-dimethyl-formamide;
for 0.25h;
With
triethylamine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 4h;
With
citric acid;
In
water; ethyl acetate; N,N-dimethyl-formamide; toluene;
pH=3 - 4;
|
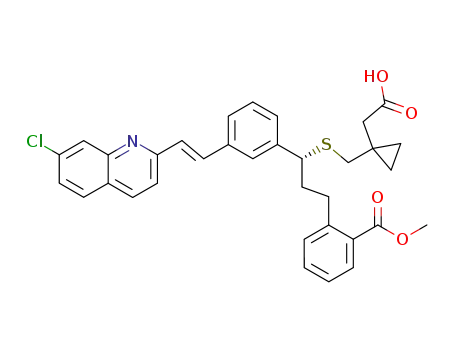
[R,E]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(methoxycarbonyl)phenyl]propyl]thio]methyl]-cyclopropane acetic acid
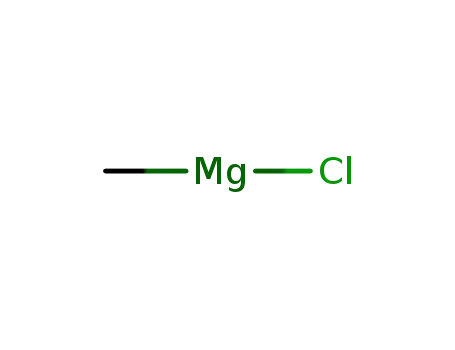
methylmagnesium chloride
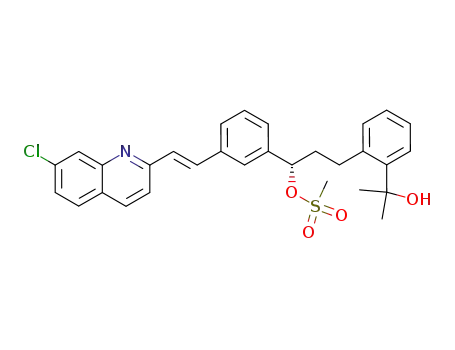
2-[2-[3(S)-[3-[(1E)-2-(7-chloroquinoline-2-yl)ethenyl]phenyl]-3-methanesulfonyloxypropyl]phenyl]-2-propanol
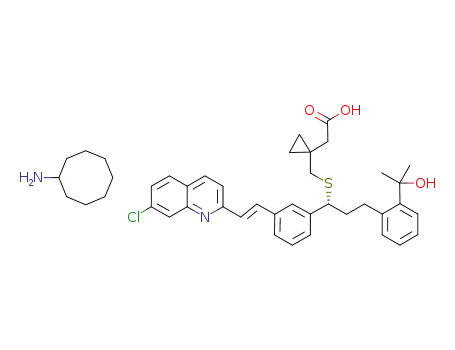
(R-(E))-1-(((1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid cyclooctylammonium salt
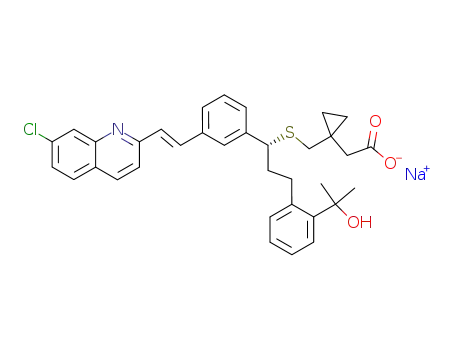
Montelukast sodium
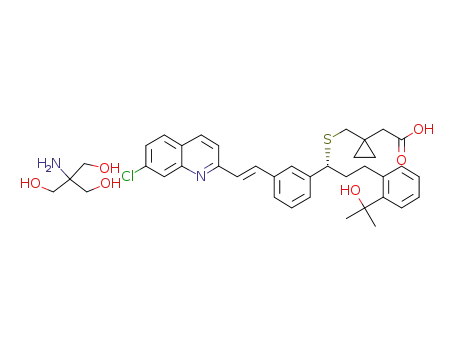
1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid tris(hydroxymethyl)aminomethane salt
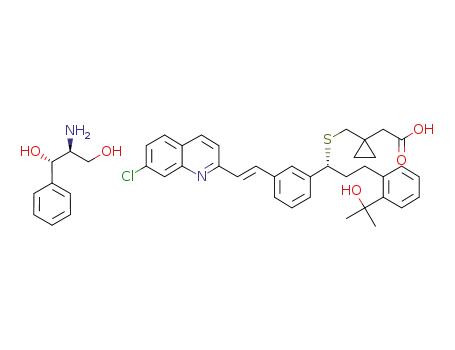
1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid L-(+)-treo-2-amino-1-phenyl-1,3-propanediol salt
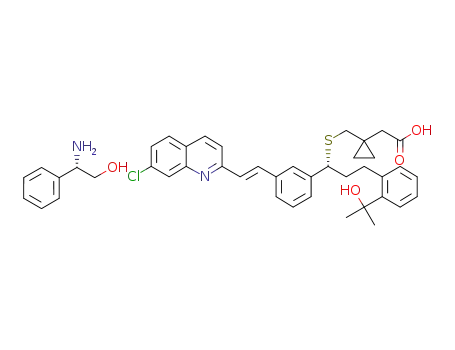
1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid L-(+)-α-phenylglycinol salt