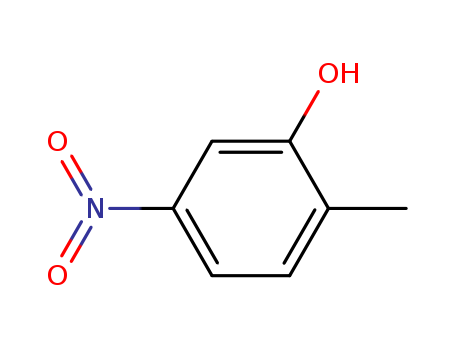

CasNo: 5428-54-6
MF: C7H7NO3
Appearance: Brown solid
InChI:InChI=1/C18H19Cl2NO4/c1-2-24-18(23)11-3-5-21(6-4-11)9-12-10-25-17-14(16(12)22)7-13(19)8-15(17)20/h7-8,10-11H,2-6,9H2,1H3
A new approach to the synthesis of immob...
Based on the promising activity of an in...
-
A highly convergent total synthesis of t...
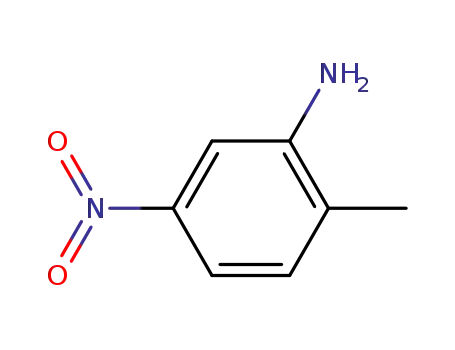
2-methyl-5-nitroaniline


2-methyl-5-nitrophenol
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; sodium nitrite;
In
water;
for 0.333333h;
Heating;
|
93% |
|
With
sulfuric acid; sodium nitrite;
In
water;
Heating;
|
87% |
|
Diazotization.Reaktion ueber mehrere Stufen;
|
|
|
Multistep reaction;
(i) aq. NaNO2, H2SO4, (ii) aq. H2SO4;
|
|
|
With
sulfuric acid; sodium nitrite;
Yield given. Multistep reaction;
1.) 2-5 deg C, 2.) heating;
|
|
|
With
sodium nitrite;
In
sulfuric acid; water;
|
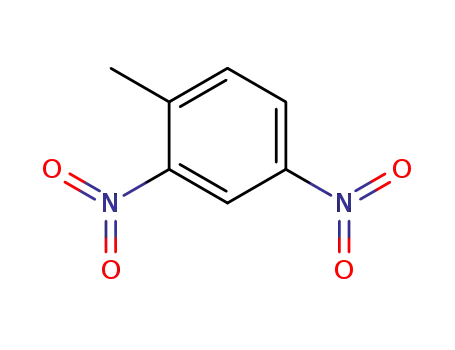
2,4-dinitrotoluene


2-methyl-5-nitrophenol

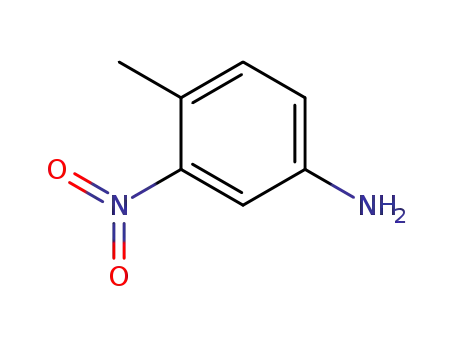
4-Methyl-3-nitroanilin


2-methyl-5-nitroaniline
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
methanol;
at 36 ℃;
for 0.25h;
under 760.051 Torr;
|

2-methyl-5-nitroaniline
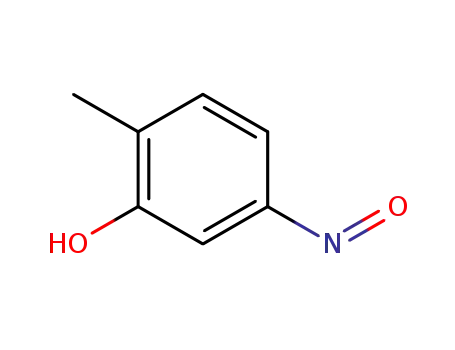
5-Nitroso-o-kresol
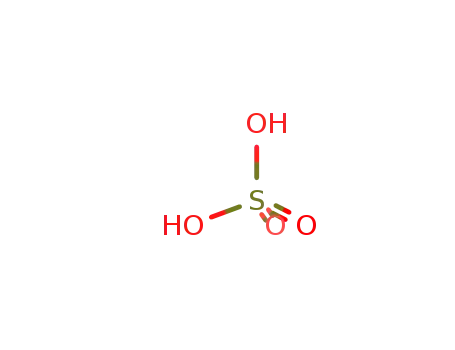
sulfuric acid
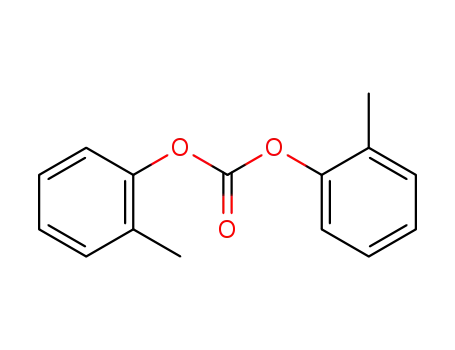
di-o-tolyl carbonate
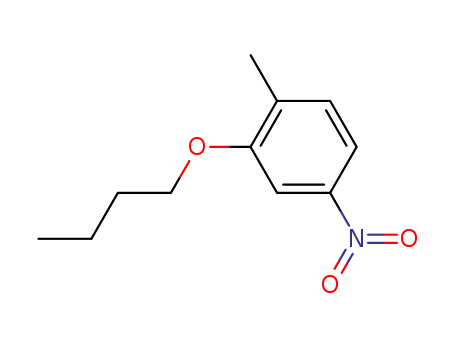
2-butoxy-1-methyl-4-nitrobenzene
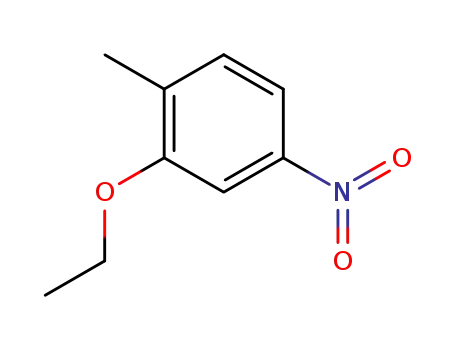
2-ethoxy-1-methyl-4-nitrobenzene
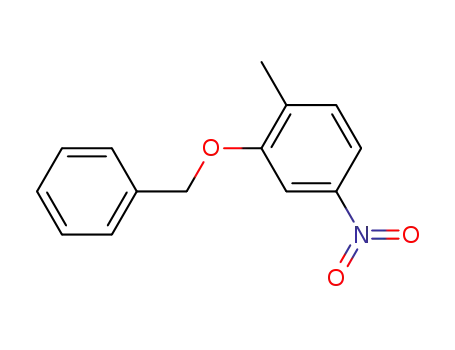
2-(benzyloxy)-1-methyl-4-nitrobenzene
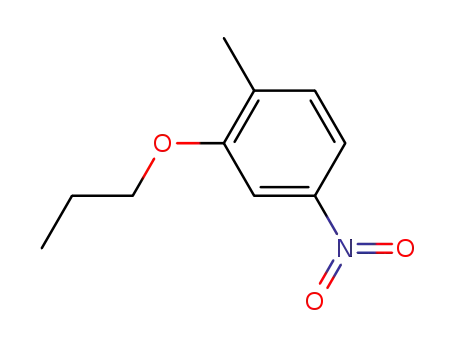
1-methyl-4-nitro-2-n-propoxybenzene