
CasNo: 1127-45-3
MF: C9H7NO2
Appearance: Yellow crystalline solid
|
Synthesis |
8-hydroxyquinoline-N-oxide was synthesized from 8-hydroxyquinoline. A stirred solution of 8-hydroxyquinoline (25.00 g, 172.2 mmol) in 550 ml of CHCl3?was cooled to 0° C., and 3 -chloroperoxybenzoic acid (40.00 g, 80% Tech. grade *0.231 mmol=0.185 mmol) was added slowly over 3 minutes. The solution was kept at 0° C. and stirred for 3 hours. During this period, the 3-chlorobenzoic acid byproduct precipitated. The 3-chlorobenzoic acid was removed by filtration and the orange filtrate was concentrated to dryness and the remaining solid was triturated with 2% NH40?H (2*200 ml). The solid was isolated on a frit and washed with H2O. |
|
General Description |
8-Quinolinol N-oxide is a toxicant which can suppress luminescence of an indicator bacterium Photobacterium phosphoreum. It forms two types of complexes with gold(III) and palladium(II): type I are ion-associated compounds containing hydrogen bond and the other is the six-membered metal chelates containing metal-oxygen bonds. |
InChI:InChI=1/C9H7NO2/c11-8-5-1-3-7-4-2-6-10(12)9(7)8/h1-6,11H
Synthesis, structure, optical absorption...
Four N-oxides, 8-quinolinol N-oxide (8-H...
A regiospecific and convergent synthesis...
Heterocyclic aromatic N-oxides often hav...
Compounds of the types ReOCl2(L)(PPh3) a...
Platelet-derived growth factor receptor ...
(Chemical Equation Presented) The direct...
Design of a paramagnetic metal binding m...
A new hexadentate chelator was synthesiz...
The first safe and efficient synthesis o...
Azodicarboxylate esters are common reage...
This paper presents a versatile reagent ...
Synthesis of 2-alkenylquinoline by reduc...
Herein we present an efficient and regio...
Catalyzed peroxidation of unsaturated li...
An improved, safe, and scalable isolatio...
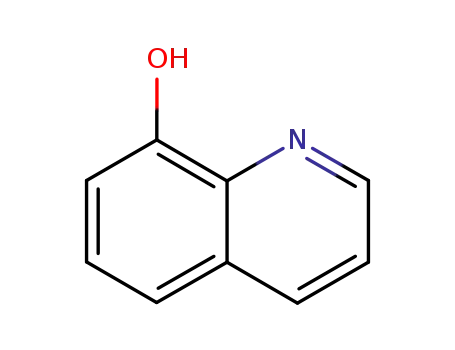
8-quinolinol

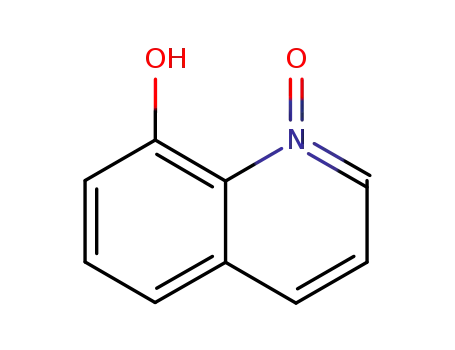
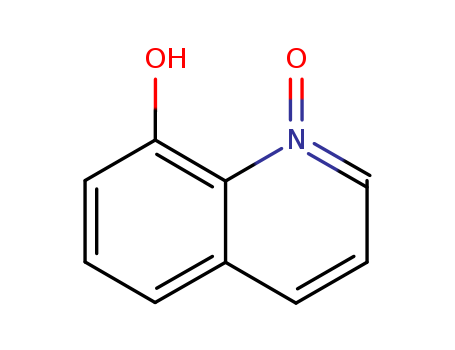
8-Hydroxyquinoline-N-oxide
| Conditions | Yield |
|---|---|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
|
100% |
|
With
dihydrogen peroxide;
In
water;
at 20 ℃;
for 4h;
Catalytic behavior;
|
98% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
Isopropyl acetate;
at 10 - 35 ℃;
for 5.83333h;
Green chemistry;
|
98.2% |
|
With
manganese dioxide; dihydrogen peroxide;
methyl rhenium trioxide;
In
dichloromethane;
|
97% |
|
With
manganese dioxide; dihydrogen peroxide;
methyl rhenium trioxide;
In
dichloromethane;
|
97% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 1.16667h;
|
97% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform;
|
95% |
|
With
dihydrogen peroxide; bis(triphenyl)oxodiphosphonium trifluoromethanesulfonate salt;
In
ethanol;
at 20 ℃;
for 0.833333h;
|
95% |
|
With
dihydrogen peroxide;
Na12[WZn3(H2O)2(ZnW9O34)2];
at 75 ℃;
for 7h;
|
94% |
|
With
dihydrogen peroxide;
VxSi4xO6.4x;
In
acetonitrile;
at 80 ℃;
for 8h;
|
92% |
|
With
phosphomolybdic acid; dihydrogen peroxide;
In
water; acetonitrile;
at 50 ℃;
for 12h;
|
89% |
|
With
dihydrogen peroxide;
In
ethanol; water;
at 24.84 ℃;
for 6h;
|
89% |
|
With
dihydrogen peroxide;
In
water;
for 0.416667h;
Sonication;
|
89% |
|
With
phosphomolybdic acid; dihydrogen peroxide;
In
acetonitrile;
at 500 ℃;
for 30h;
|
85.9% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 3h;
|
85% |
|
With
oxygen; isobutyraldehyde;
In
1,2-dichloro-ethane;
at 30 ℃;
for 22h;
|
84% |
|
With
peracetic acid;
In
dichloromethane; water;
at 6 - 20 ℃;
for 3h;
|
84.5% |
|
With
(dimethyldioctadecylammonium)8 [HBW11O39]; dihydrogen peroxide;
In
tert-butyl alcohol;
at 65 ℃;
for 5h;
|
82% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform;
at 0 ℃;
|
77% |
|
With
peracetic acid; acetic acid;
In
dichloromethane; water;
at 0 - 20 ℃;
for 5h;
|
76% |
|
With
peracetic acid;
In
dichloromethane; water;
at 0 - 20 ℃;
for 5.25h;
|
76% |
|
With
1,1-Dibromoethane; tetradecafluorohexane; 3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
for 72h;
|
75% |
|
With
dihydrogen peroxide;
In
acetic acid;
Heating;
|
70% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
Cooling with ice;
|
70.3% |
|
With
dihydrogen peroxide; acetic acid;
for 0.333333h;
Heating;
|
58% |
|
With
dihydrogen peroxide;
In
acetic acid;
for 0.666667h;
Reflux;
|
51% |
|
With
dihydrogen peroxide; acetic acid;
at 70 ℃;
for 16h;
|
40% |
|
With
dihydrogen peroxide;
In
acetic acid;
for 72h;
|
38% |
|
With
dihydrogen peroxide; acetic acid;
at 70 ℃;
for 16h;
|
33% |
|
With
dihydrogen peroxide; acetic acid;
at 70 - 80 ℃;
for 11h;
|
25% |
|
With
Perbenzoic acid;
In
1,4-dioxane; water;
at 20 ℃;
Rate constant;
Kinetics;
Thermodynamic data;
Ea, ΔS%;
|
|
|
With
water; dihydrogen peroxide; acetic acid;
|
|
|
With
dihydrogen peroxide;
In
acetic acid;
Heating;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
|
|
|
With
dihydrogen peroxide; acetic acid;
In
water;
at 70 ℃;
|
|
|
With
dihydrogen peroxide;
In
1,4-dioxane; water;
at 65 ℃;
for 6h;
|
95 %Chromat. |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 1.16667h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
for 2h;
Cooling with ice;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 12h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
|
|
|
With
Candida antarctica lipase B; D-glucose; glucose oxidase from A. Niger; oxygen;
In
aq. phosphate buffer; ethyl acetate;
at 20 ℃;
for 1h;
Green chemistry;
|
|
|
With
baeyer?villiger monooxygenase like enzyme in Lysobacter antibioticus OH13;
at 30 ℃;
Enzymatic reaction;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
|
|
|
With
dihydrogen peroxide;
perfluoroketone-silicate;
In
acetonitrile;
at 80 ℃;
for 24h;
|
20 % Chromat. |
|
With
sodium tungstate (VI) dihydrate; dihydrogen peroxide;
In
water;
at 75 ℃;
for 5h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
|
|
With
dihydrogen peroxide; acetic acid;
at 70 ℃;
for 36h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 0.5h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 15h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 28 ℃;
for 24h;
|
|
|
With
dihydrogen peroxide; acetic acid;
In
water;
at 75 ℃;
for 36h;
|

8-quinolinol

3-chloroperbenzoic acid (MCPBA)


8-Hydroxyquinoline-N-oxide

![8-[(2,3,4,5-Tetrahydro-2-methyl-4-methylene-5-oxo-2-furanyl)methoxy]-2(1H)-quinolinone](/upload/2025/3/92aa9105-1a1c-400b-ada4-e114b24ad5c8.png)
8-[(2,3,4,5-Tetrahydro-2-methyl-4-methylene-5-oxo-2-furanyl)methoxy]-2(1H)-quinolinone
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
|
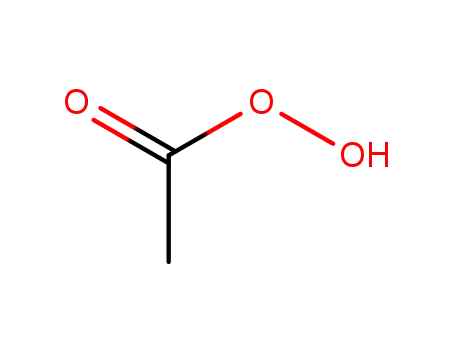
peracetic acid

8-quinolinol
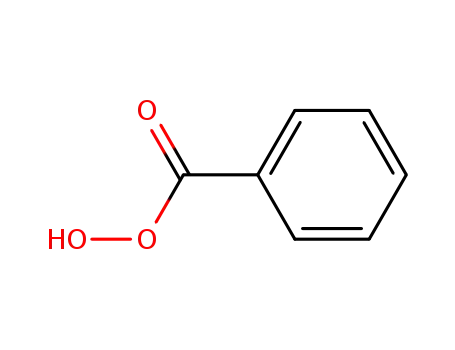
Perbenzoic acid
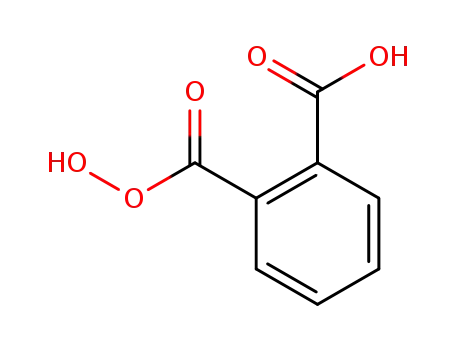
monoperoxyphthalic acid
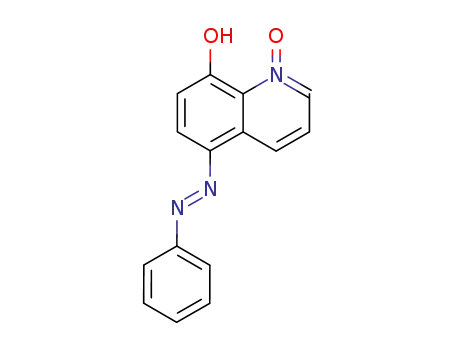
1-oxy-5-phenylazo-quinolin-8-ol
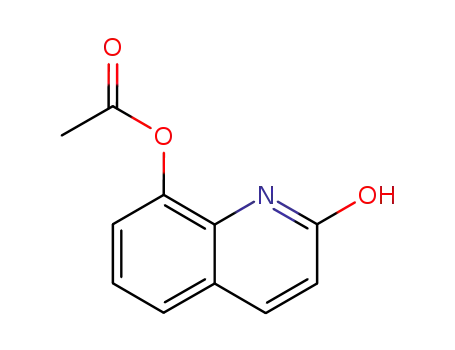
2-hydroxyquinolin-8-yl acetate
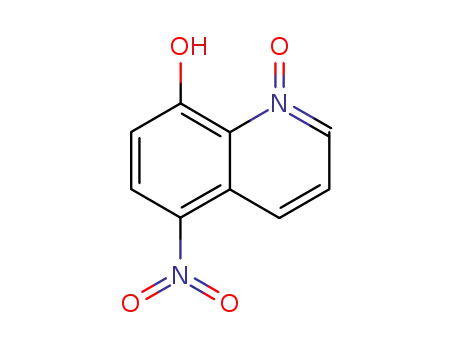
5-nitro-8-hydroxyquinoline-N-oxide
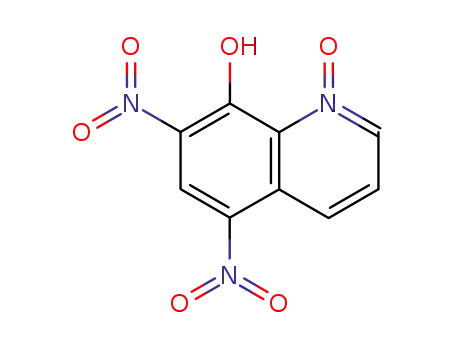
5,7-dinitro-8-hydroxyquinoline-N-oxide