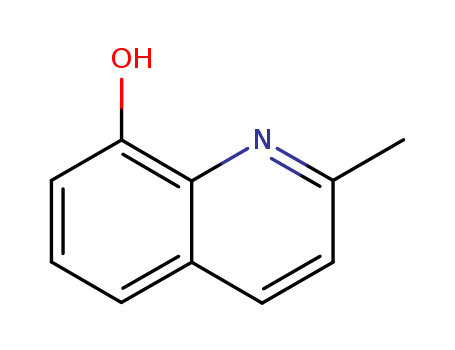

CasNo: 826-81-3
MF: C10H9NO
Appearance: beige to brown crystalline powder
|
Preparation |
8-hydroxyquinaldine is obtained from the reaction of 2-aminophenol with crotonaldehyde. Mix 2-aminophenol and 2-Nitrophenol homogeneously and add hydrochloric acid. Add crotonaldehyde under stirring. Heat for 6h and leave overnight. Distill the reacted o-nitrophenol by water distillation, and add sodium hydroxide solution to the residual solution to make it weakly basic. Then add powdered carbonyls for saturation, and distill 8-Hydroxyquinaldine by water distillation, and then distill the crude product under reduced pressure and recrystallize it with ethanol to obtain 8-hydroxyquinaldine. |
|
General Description |
2-Methyl-8-quinolinol is a methyl substituted quinolinol derivative that shows fungicidal property. It can also undergo complexation with transition metal complexes. |
|
Purification Methods |
Crystallise the quinoline from EtOH or aqueous EtOH. Its solubility at 20o in H2O is 0.366g/L, and in CHCl3 it is 466g/L. It complexes with many metals. [Beilstein 21 H 106, 21 III/IV 2132.] |
InChI:InChI=1/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3
-
Herein, an efficient and selective nicke...
The invention belongs to the technical f...
Organic light-emitting diodes (OLEDs) pr...
Herein, we disclose the development of a...
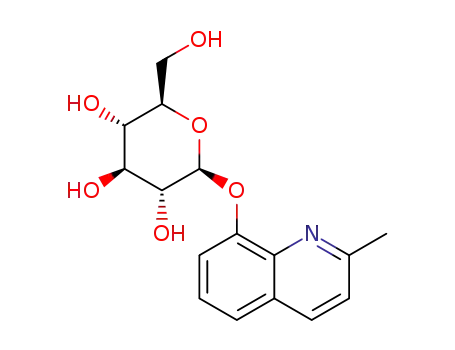
2-methyl-8-quinolinyl-β-D-glucopyranoside

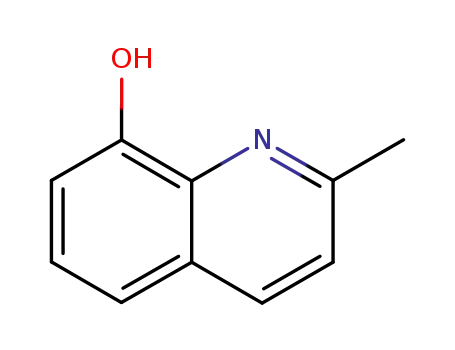
2-methyl-8-quinolinol

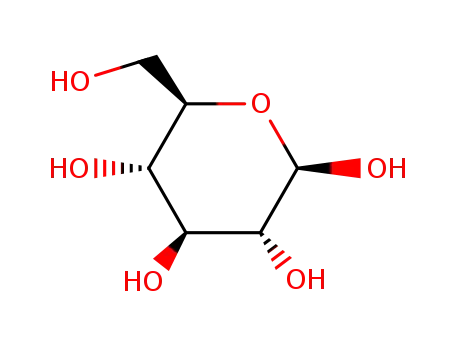
β-D-glucose
| Conditions | Yield |
|---|---|
|
With
β-glucosidase from almonds; water;
In
aq. phosphate buffer;
at 37 ℃;
for 4h;
pH=7.4;
Reagent/catalyst;
Enzymatic reaction;
|
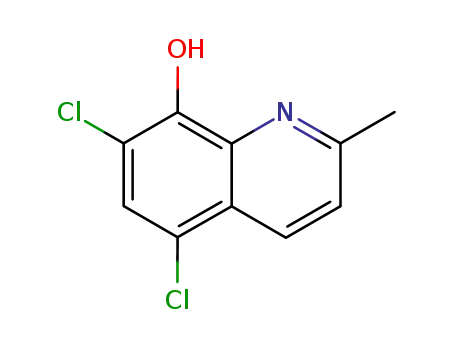
chloroquinaldol


2-methyl-8-quinolinol
| Conditions | Yield |
|---|---|
|
With
palladium hydroxide 10 wt. % on activated carbon; hydrogen; triethylamine;
In
ethanol;
at 43 ℃;
for 6h;
under 9750.98 - 11251.1 Torr;
Solvent;
Reagent/catalyst;
Pressure;
|
62.67% |
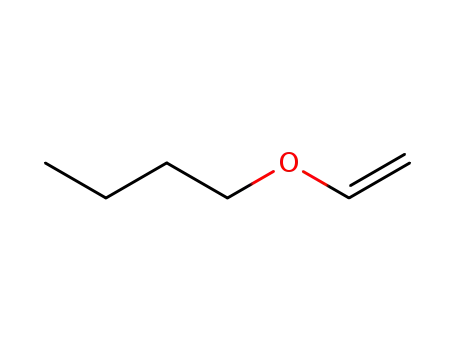
-butyl vinyl ether
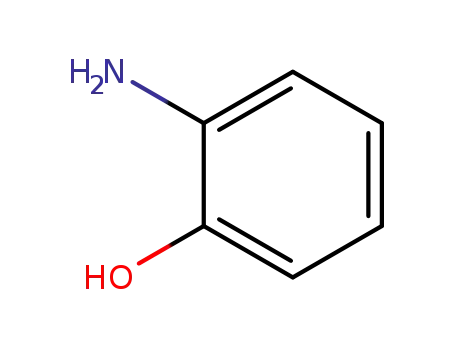
2-amino-phenol
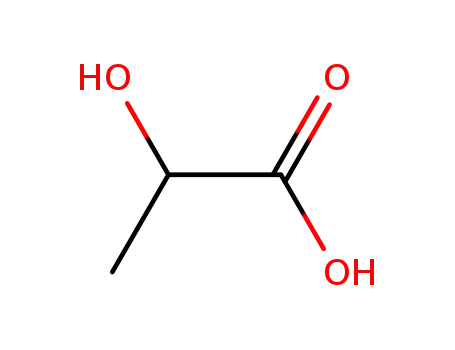
LACTIC ACID

2-hydroxynitrobenzene
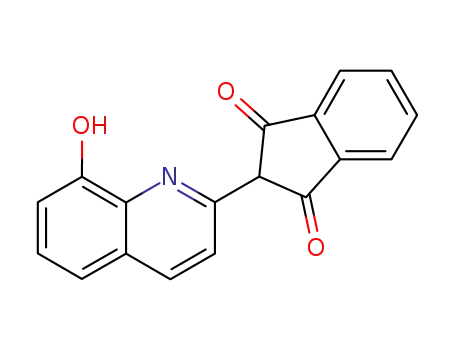
2-(8-hydroxy-quinolin-2-yl)-indan-1,3-dione
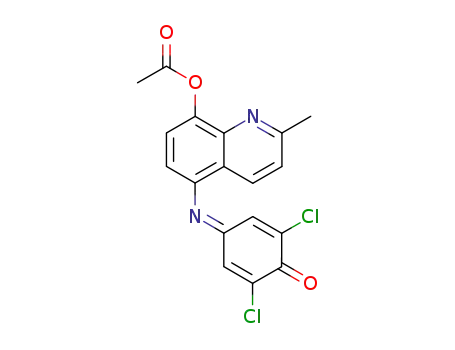
4-(8-acetoxy-2-methyl-[5]quinolylimino)-2,6-dichloro-cyclohexa-2,5-dienone
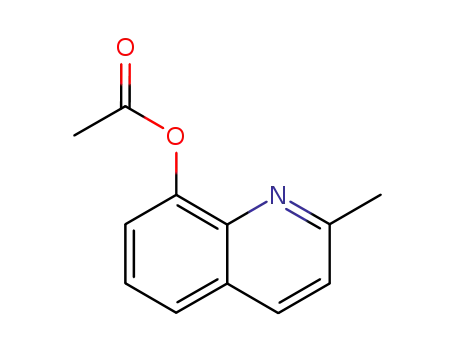
2‐methyl‐8‐acetoxyquinoline
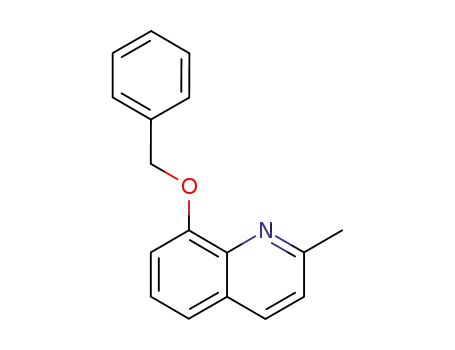
2-methyl-8-(benzyloxy)quinoline