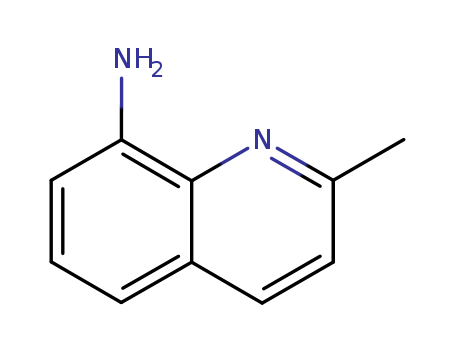

CasNo: 18978-78-4
MF: C10H10N2
Appearance: Yellow Crystals
|
Synthesis Reference(s) |
Tetrahedron, 52, p. 2937, 1996 DOI: 10.1016/0040-4020(95)01118-8 |
InChI:InChI=1/C10H10N2/c1-7-5-6-8-3-2-4-9(11)10(8)12-7/h2-6H,11H2,1H3
Herein, we report the synthesis of palla...
In recent years, resistance to the antim...
A convenient synthesis for 4- and 7-n-un...
Ru-PNN pincer catalysts of the general f...
A new synthetic route to amidoquinoline ...
Starting from o-anisidine, alkylated 8-h...
Novel 7,16-bis(8-amino-2-quinolinylmethy...
The systematic SAR study of a "caging" g...
A multi-functionalized ligand, based on ...
The hydrogenation of heterocyclic nitroa...
A mild transition-metal- and photosensit...
The invention relates to a method for re...
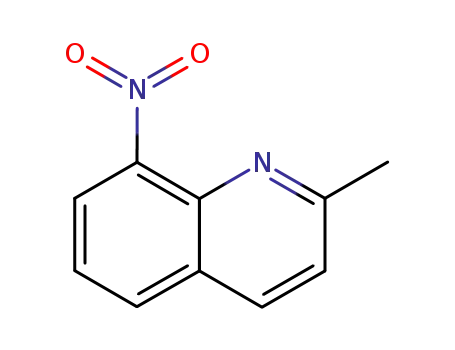
8-nitroquinaldine

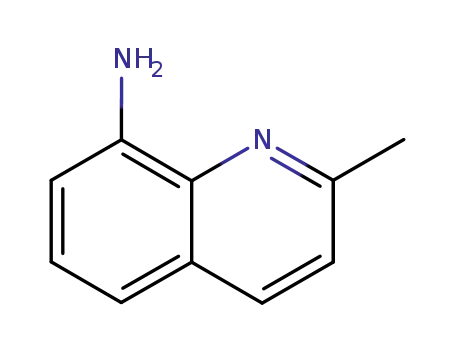
2-methyl-8-aminoquinoline
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
for 2h;
|
99% |
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
ethanol;
for 5h;
|
99% |
|
With
hydrogen;
In
tetrahydrofuran;
at 60 ℃;
under 15001.5 Torr;
Flow reactor;
|
99.7% |
|
With
tetrahydroxydiboron; 5%-palladium/activated carbon; water;
In
acetonitrile;
at 50 ℃;
for 24h;
|
97% |
|
With
tetrahydroxydiboron; palladium on activated charcoal; water;
In
acetonitrile;
at 50 ℃;
for 24h;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
97% |
|
With
hydrazine hydrate;
palladium on activated charcoal;
In
methanol;
for 3h;
Heating;
|
95% |
|
With
hydrogen;
palladium on activated charcoal;
|
95% |
|
With
hydrogen iodide;
at 90 ℃;
for 2h;
|
95% |
|
With
hydrogenchloride; iron;
In
ethanol; water; acetic acid;
for 0.333333h;
Reflux;
|
93% |
|
With
tetrahydroxydiboron; copper diacetate;
In
acetonitrile;
at 80 ℃;
for 24h;
chemoselective reaction;
Schlenk technique;
|
90% |
|
With
tris(bipyridine)ruthenium(II) dichloride hexahydrate; ascorbic acid;
In
methanol; water;
at 20 ℃;
for 3.5h;
chemoselective reaction;
Schlenk technique;
Inert atmosphere;
Irradiation;
Green chemistry;
|
90% |
|
8-nitroquinaldine;
With
hydrogen iodide;
In
water;
at 90 ℃;
for 2h;
With
sodium hydrogencarbonate;
In
water;
at 20 ℃;
|
89% |
|
With
ethanol; acetic acid;
for 4h;
Reflux;
|
88% |
|
With
iron; acetic acid;
|
83% |
|
With
hydrogen; palladium(II) hydroxide;
|
80% |
|
With
iron(III) chloride hexahydrate; hydrazine hydrate;
In
methanol;
Reflux;
|
68% |
|
With
phenylsilane; triphenylphosphine; sodium iodide;
In
chloroform;
at 60 ℃;
for 72h;
Inert atmosphere;
Irradiation;
|
62% |
|
With
tetrahydroxydiboron; water;
at 80 ℃;
for 8h;
|
52% |
|
With
ammonium sulfide;
In
ethanol;
for 2h;
Heating;
|
45% |
|
With
hydrogenchloride; tin(ll) chloride;
|
|
|
With
methanol; nickel;
Hydrogenation;
|
|
|
With
hydrogen;
palladium on activated charcoal;
In
ethanol;
at 20 ℃;
|
|
|
With
palladium on activated charcoal; hydrogen;
In
1,4-dioxane;
for 12h;
|
|
|
With
tin(ll) chloride;
Acidic conditions;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
1,4-dioxane;
for 12h;
|
|
|
With
tin(ll) chloride;
In
ethanol;
at 70 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
With
tin(ll) chloride;
In
ethanol;
at 70 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 50 ℃;
for 4h;
under 760.051 Torr;
Inert atmosphere;
|
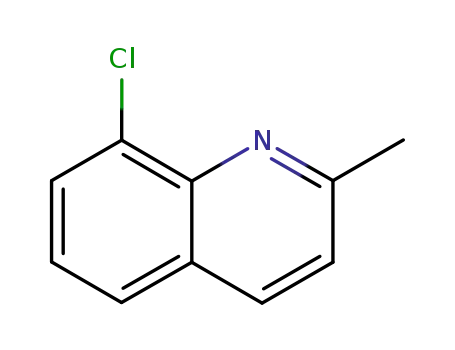
8-chloroquinaldine


2-methyl-8-aminoquinoline
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide; potassium phosphate; copper(l) iodide; N1,N2-bis(5-methyl-[1,1'-biphenyl]-2-yl)oxalamide;
In
water; dimethyl sulfoxide;
at 120 ℃;
for 24h;
Inert atmosphere;
|
78% |
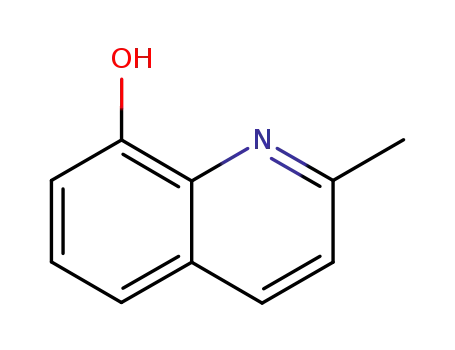
2-methyl-8-quinolinol
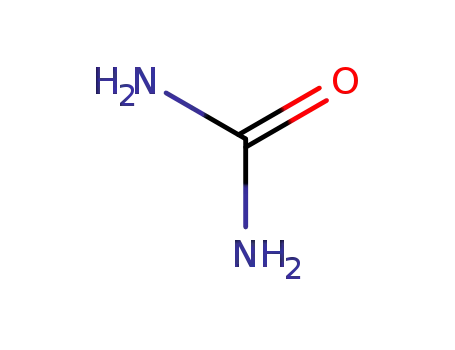
urea

8-nitroquinaldine
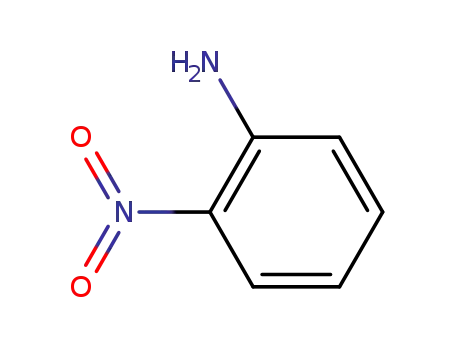
2-nitro-aniline
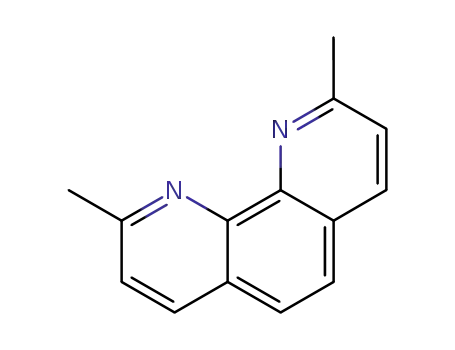
2.9-dimethyl-1,10-phenanthroline
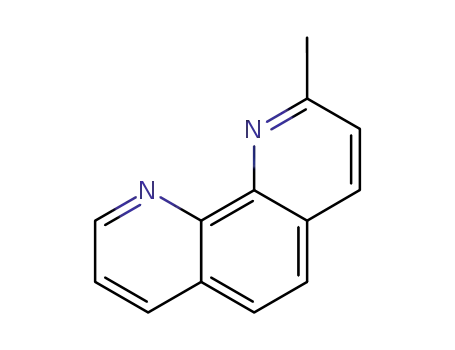
2-methyl-1,10-phenanthroline
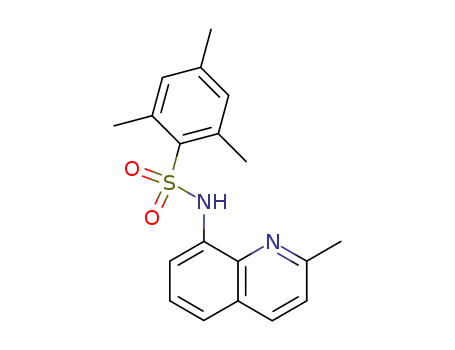
2-methyl-8-mesitylenesulfonamidoquinoline
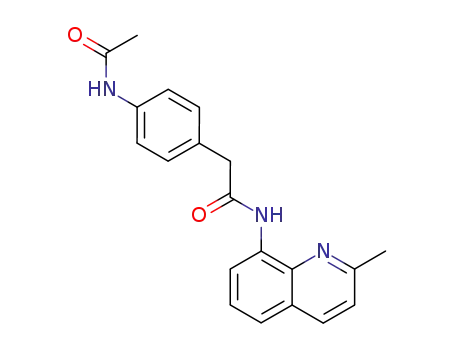
2-(4-Acetylamino-phenyl)-N-(2-methyl-quinolin-8-yl)-acetamide