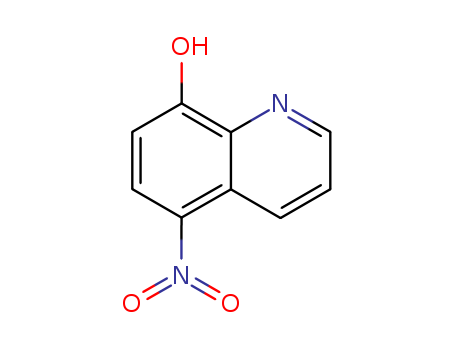

CasNo: 4008-48-4
MF: C9H6N2O3
Appearance: ochre-yellow to brownish crystalline powder
|
World Health Organization (WHO) |
Nitroxoline, a urinary antiseptic, was introduced in the mid-1960s. By the early 1970s long-term animal studies revealed the development of cataracts in rats and, although no serious adverse effects had been reported in man, the drug was withdrawn in at least two countries. Preparations containing nitroxoline remain widely available. |
|
Biochem/physiol Actions |
8-Hydroxy-5-nitroquinoline is an effective anti-microbial and anti-cancer agent. It is an effective drug for the treatment of urinary tract infections due to gram negative bacilli. |
|
in vitro |
the machnistic study showed that nitroxoline, in the treatment of acute or recurrent urinary tract infections caused by escherichia coli, could be decreased in the presence of mg2+ and mn2+ but not ca2+. moreover, with the divalent metal ions, a shift in the nitroxoline a448 indicated the formation of drug-ion complexes and a clear correlation was observed between the chelating property and antibacterial activity of nitroxolinet. in addition, it was found that the uptake was energy-independent and with biphasic kinetics: a rapid cell association phase and then a slower increase of cell- nitroxoline association [1]. |
|
in vivo |
previous animal study showed that nitroxoline suspension with tween-80 in a could decrease the tone of the rat and guinea-pig ileum and diminish their peristalsis. moreover, when administered orally in a dose 50 mg/kg to rats, nitroxoline was able to inhibit the agar-, serotonin-, as well as carrageenin-induced edemas of the rat paws without affecting the response to subplantar histamine injection [2]. |
|
references |
[1] pelletier c,prognon p,bourlioux p. roles of divalent cations and ph in mechanism of action of nitroxoline against escherichia coli strains. antimicrob agents chemother.1995 mar;39(3):707-13.[2] zaks as,zil'ber al,kapitonenko ta. spasmolytic and anti-inflammatory activity of 8-hydroxyquinolines. farmakol toksikol.1984 sep-oct;47(5):44-7.[3] lambert-zechovsky n,lévêque b,bingen e,pillion g,chapelle j,mathieu h. clinical study and effect of nitroxoline on fecal flora in childrenpathol biol (paris).1987 may;35(5):669-72. |
|
Definition |
ChEBI: A monohydroxyquinoline in which the hydroxy group is positioned at C-8 with a nitro group trans to it at C-5. |
|
Brand name |
5-nitrok;Dovenix;Entercol;Enterocol;Isinok;Nicene;Nikinol;Nikopet;Noxine;Trodax;Uro-coli. |
InChI:InChI=1/C9H6N2O3/c12-8-4-3-7(11(13)14)9-6(8)2-1-5-10-9/h1-5,12H
Two angular phenothiazines and one angul...
The crystal structures of 8-hydroxy-5-ni...
A series of selenium-containing clioquin...
The synthesis of angular 1-azabenzo[a]ph...
Phytopathogenic fungi have become a seri...
Organic light-emitting diodes (OLEDs) pr...
Base mediated condensation reaction betw...
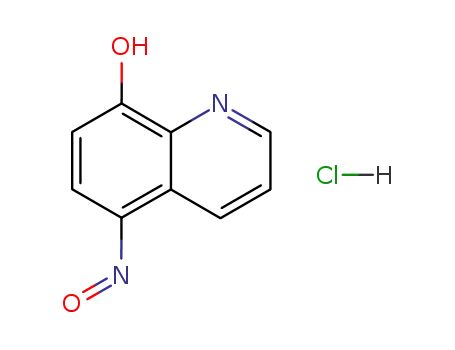
5-Nitroso-8-hydroxyquinoline hydrochloride

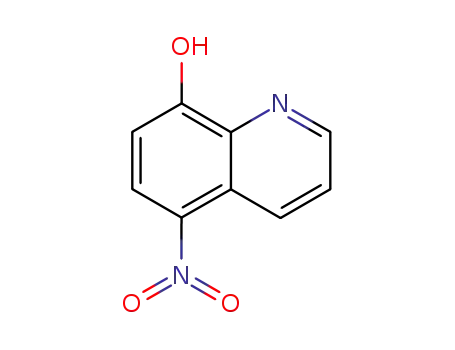
nitroxoline
| Conditions | Yield |
|---|---|
|
With
nitric acid;
In
water;
at 17 ℃;
for 1.41667h;
Inert atmosphere;
Cooling with ice;
|
90.1% |
|
With
nitric acid;
|
18.6 g |
|
With
nitric acid;
In
water;
at 17 ℃;
for 1.25h;
|
|
|
With
nitric acid;
|
|
|
5-Nitroso-8-hydroxyquinoline hydrochloride;
With
nitric acid;
In
water;
With
potassium hydroxide;
With
acetic acid;
|
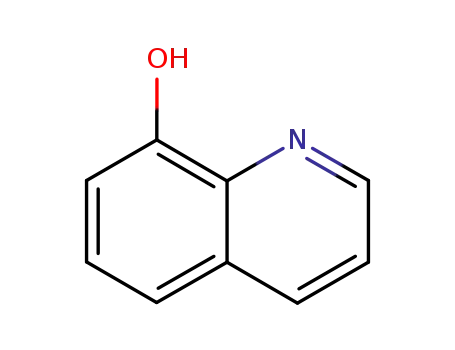
8-quinolinol


nitroxoline
| Conditions | Yield |
|---|---|
|
8-quinolinol;
With
sulfuric acid; sodium nitrite;
In
water;
at 15 - 18 ℃;
With
nitric acid;
In
water;
at 20 ℃;
|
80% |
|
With
copper(II) nitrate;
In
water; acetic acid;
for 0.00833333h;
microwave irradiation;
|
70% |
|
Multi-step reaction with 2 steps
1: 96 percent / H2SO4; aq. NaNO2 / 3 h / 18 - 20 °C
2: 23.44 g / HNO3 / acetic acid / 2 h / 25 - 30 °C
With
sulfuric acid; nitric acid; sodium nitrite;
In
acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 95 percent / NaNO2; HCl
2: 18.6 g / HNO3
With
hydrogenchloride; nitric acid; sodium nitrite;
|
|
|
Multi-step reaction with 3 steps
1: alcoholic aqueous NaOH-solution
2: nitric acid / 0 °C / dann Erhitzen auf dem Wasserbad
3: concentrated hydrochloric acid / 180 - 190 °C
With
hydrogenchloride; sodium hydroxide; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
8-quinolinol;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 ℃;
for 0.5h;
With
nitric acid;
In
acetic acid;
at 35 - 37 ℃;
for 0.666667h;
Cooling with ice;
|
|
|
8-quinolinol;
With
sodium nitrite;
With
nitric acid;
|
|
|
8-quinolinol;
With
sulfuric acid; sodium nitrite;
In
water;
at 15 - 18 ℃;
With
nitric acid;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: sodium nitrite; hydrogenchloride / water / 0 - 4 °C
2: nitric acid / water / 1.25 h / 17 °C
With
hydrogenchloride; nitric acid; sodium nitrite;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: hydrogenchloride; sodium nitrite / water / 1 h / 0 - 4 °C
2: nitric acid
With
hydrogenchloride; nitric acid; sodium nitrite;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: sodium nitrite; hydrogenchloride / water / 0 - 5 °C
2: nitric acid / water
With
hydrogenchloride; nitric acid; sodium nitrite;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid; sodium nitrite
2: sulfuric acid; nitric acid
With
sulfuric acid; nitric acid; sodium nitrite;
|
|
|
Multi-step reaction with 2 steps
1: hydrogenchloride; sodium nitrite / water / 1 h / 0 °C
2: nitric acid / water / 1.25 h / 17 °C
With
hydrogenchloride; nitric acid; sodium nitrite;
In
water;
|
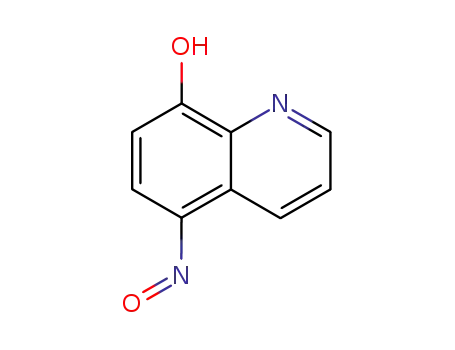
5-nitroso-8-hydroxyquinoline
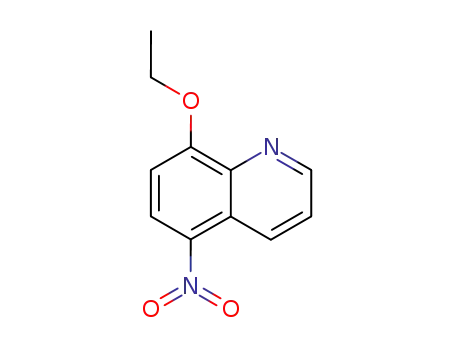
8-ethoxy-5-nitroquinoline
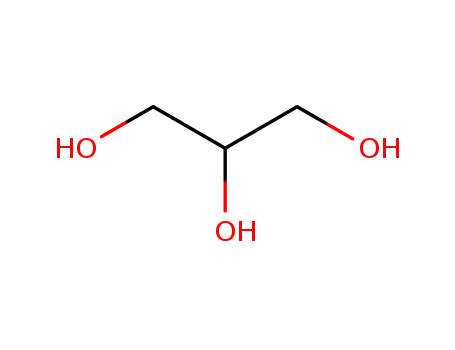
glycerol
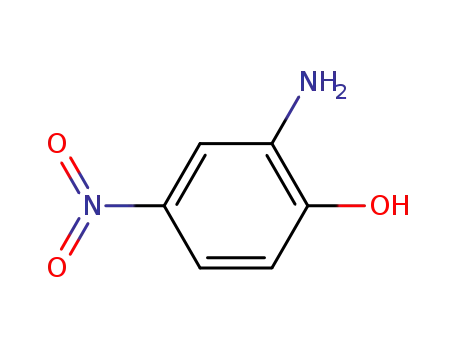
2-hydroxy-5-nitroaniline
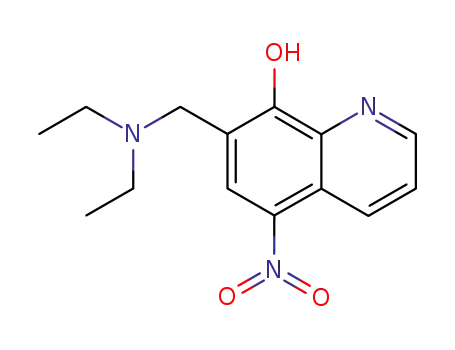
7-diethylaminomethyl-5-nitro-quinolin-8-ol
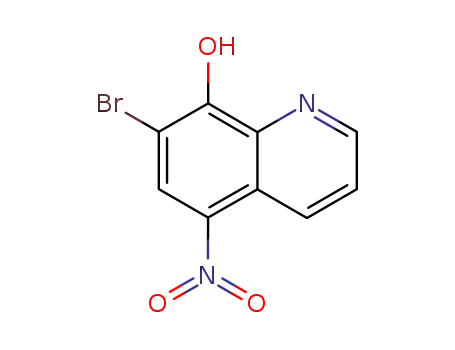
7-bromo-8-hydroxy-5-nitroquinoline
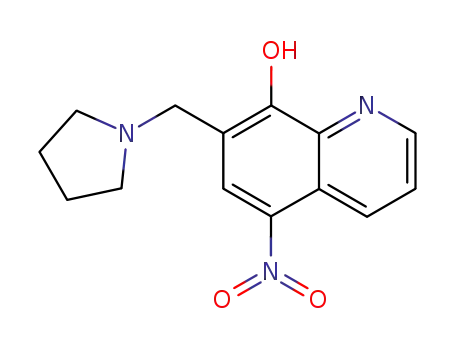
5-nitro-7-(pyrrolidin-1-ylmethyl)quinolin-8-ol
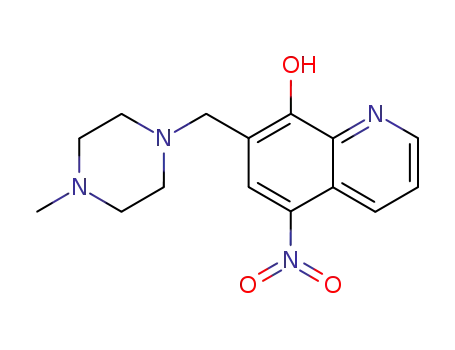
7-((4-methylpiperazin-1-yl)methyl)-5-nitroquinolin-8-ol