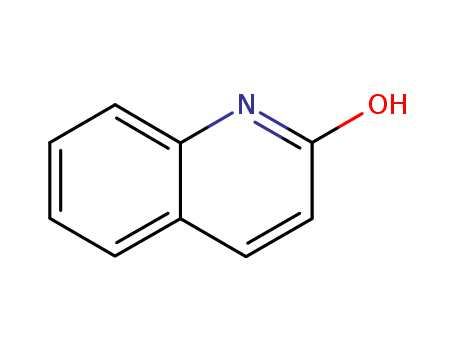

CasNo: 59-31-4
MF: C9H7NO
Appearance: white to light purple or purple-brownish powder
|
Synthesis Reference(s) |
Synthesis, p. 739, 1975 DOI: 10.1055/s-1975-23918 |
|
Purification Methods |
Crystallise it from MeOH. It has m 200-201o after sublimation in a vacuum. The picrate has m 132o after crystallisation from Et2O. [Gibson et al. J Chem Soc 4340 1955, Beilstein 21 III/IV 1057, 21/8 V 217.] |
|
Definition |
ChEBI: A quinolone that is 1,2-dihydroquinoline substituted by an oxo group at position 2. |
|
General Description |
2-Hydroxyquinoline is a specific inhibitor of plaque paraoxonase1 (PON1). |
InChI:InChI=1/C9H7NO/c11-9-6-5-7-3-1-2-4-8(7)10-9/h1-6H,(H,10,11)
-
Photo-responsive modifications and photo...
The value of catalytic dehydrogenation o...
An iron-catalyzed α,β-dehydrogenation of...
Quinolin-2(1H)-ones are one of the impor...
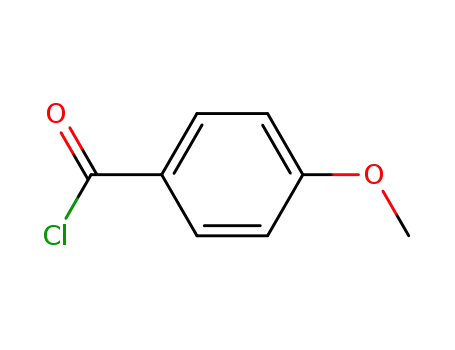
4-methoxy-benzoyl chloride

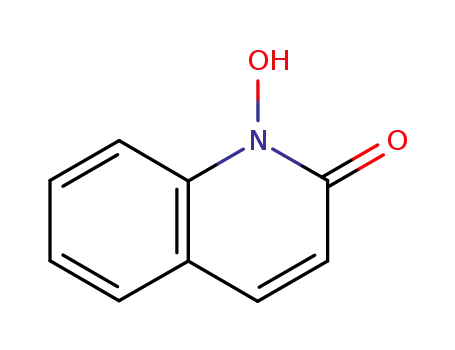
1-hydroxycarbostyril

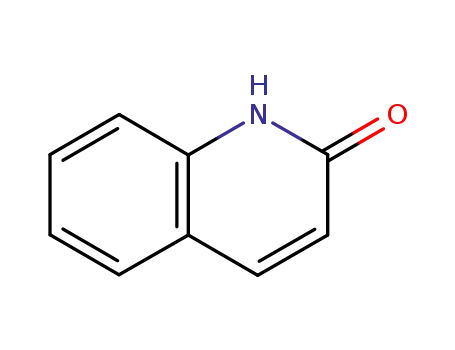
2-quinolone

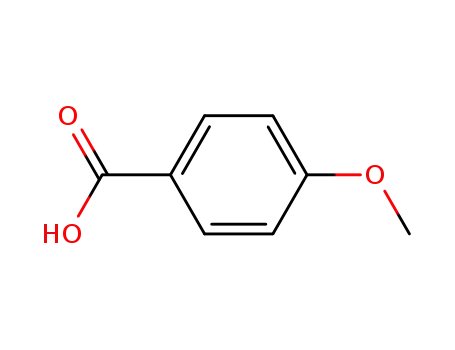
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: triethylamine / dichloromethane / 12 h / 20 °C
2: water; methanol / UV-irradiation; Photolysis
With
triethylamine;
In
methanol; dichloromethane; water;
|
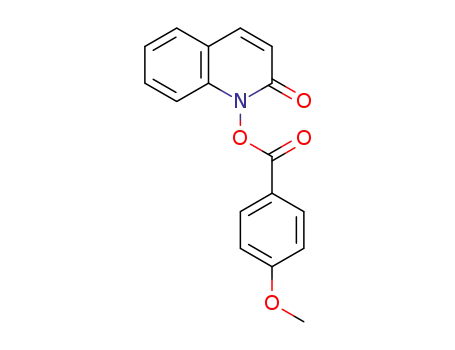
1-(p-methoxybenzoyloxy)-2(1H)-quinolone


2-quinolone


4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
In
methanol; water;
Quantum yield;
UV-irradiation;
Photolysis;
|
85% |
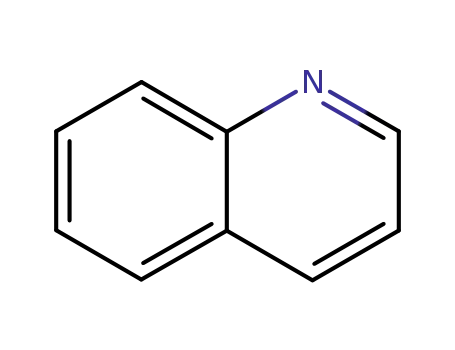
quinoline
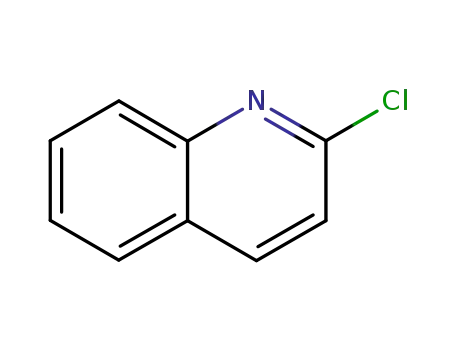
2-Chloroquinoline
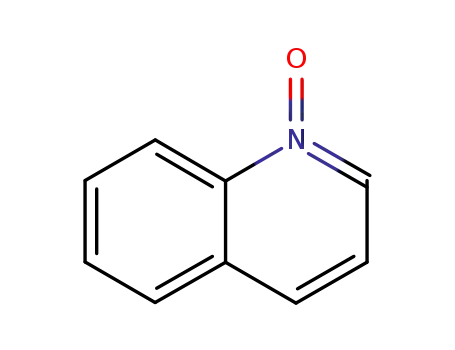
Quinoline N-oxide
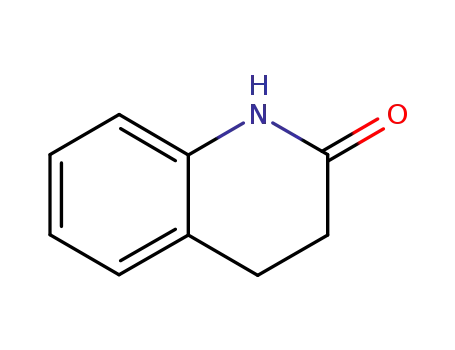
3,4-dihydro-2(1H)-quinolone
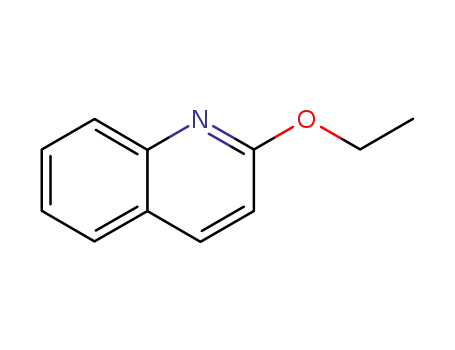
2-ethoxyquinoline
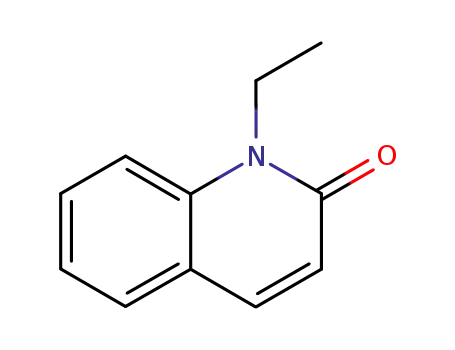
1-Ethyl-1,2-dihydrochinolin-2-on

2-Chloroquinoline
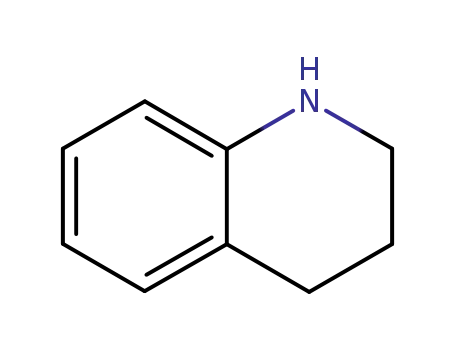
1,2,3,4-tetrahydroisoquinoline