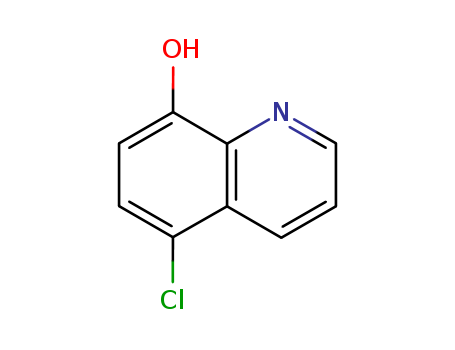

CasNo: 130-16-5
MF: C9H6ClNO
Appearance: light green to grey powder
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 70, p. 8590, 2005 DOI: 10.1021/jo051191x |
|
in vitro |
Cloxiquine (cloxyquin) exhibits antituberculosis activities, with MICs ranging from 0.062 to 0.25 μg/mL against 9 standard strains and 150 Mycobacterium tuberculosis .Cloxiquine (0.5-10 μM; 24 h) suppresses both B16F10 and A375 cell growth in a dose-dependent manner.|Cloxiquine (0.5-10 μM; 24 h) inhibits the migration of B16F10 and A375 cells.Cloxiquine (0.5-2.5 μM; 24 h) suppresses glycolysis in B16F10 cells. |
|
in vivo |
Cloxiquine (5-25 mg/kg; i.p. daily for 8 d) suppresses tumor growth in a mouse B16F10 melanoma xenograft model.Cloxiquine (5-25 mg/kg; i.p. daily for 14 d) suppresses tumor metastasis in mouse B16F10 melanoma lung metastatic model. |
InChI:InChI=1/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H
A convenient and nonreductive deiodinati...
8-Hydroxyquinoline derivatives are metal...
An operationally simple and metal-free p...
Organic light-emitting diodes (OLEDs) pr...
The invention discloses a preparation me...
A robust synthetic method has been devel...
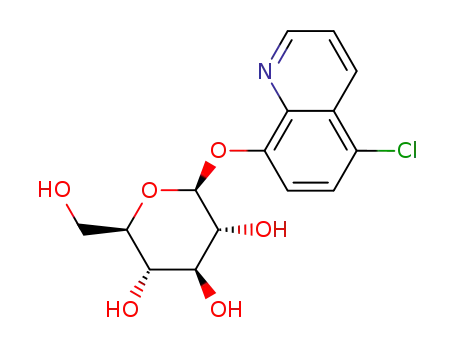
5-chloro-8-quinolinyl-β-D-glucopyranoside

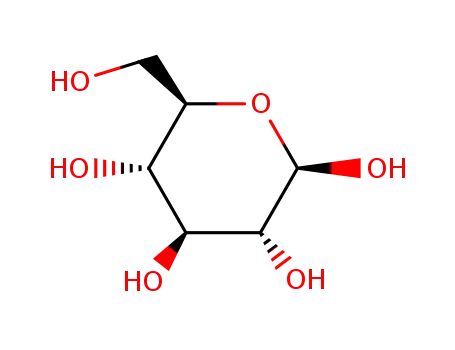
β-D-glucose

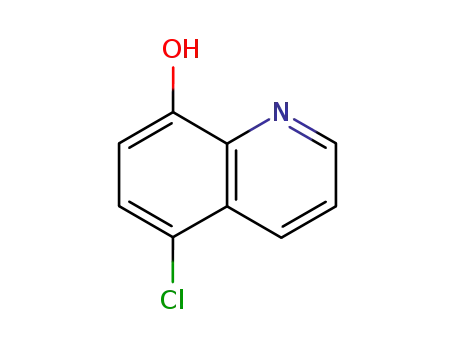
5-Chloro-8-hydroxyquinoline
| Conditions | Yield |
|---|---|
|
With
β-glucosidase from almonds; water;
In
aq. phosphate buffer;
at 37 ℃;
for 0.666667h;
pH=7.4;
Reagent/catalyst;
Enzymatic reaction;
|
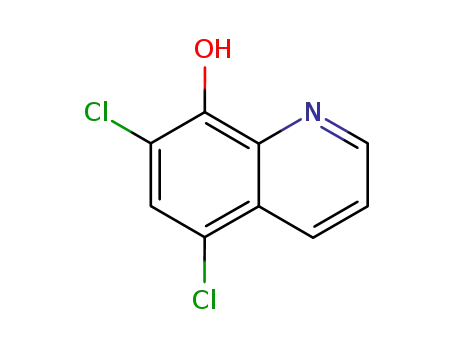
chloroxine

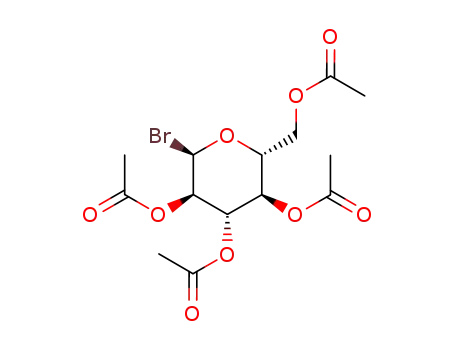
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide


β-D-glucose


5-Chloro-8-hydroxyquinoline
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: potassium carbonate; tetrabutylammomium bromide / methanol; water; dichloromethane / 68 h / 20 °C
2: β-glucosidase from almonds; water / aq. phosphate buffer / 0.67 h / 37 °C / pH 7.4 / Enzymatic reaction
With
β-glucosidase from almonds; tetrabutylammomium bromide; water; potassium carbonate;
In
methanol; aq. phosphate buffer; dichloromethane; water;
1: |Michael Addition;
|
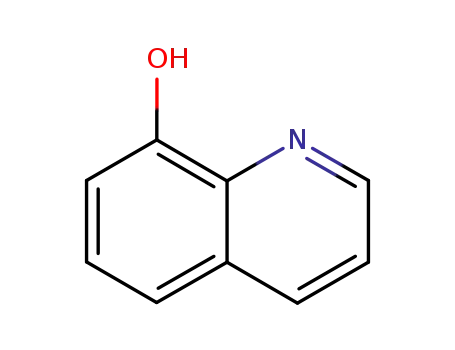
8-quinolinol
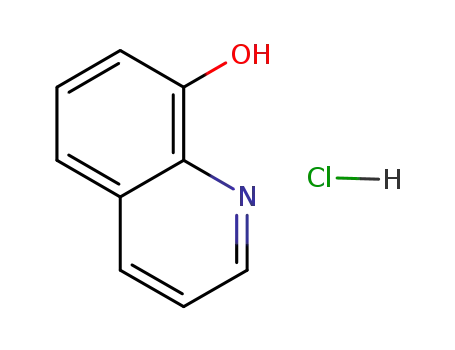
8-Hydroxyquinoline hydrochloride
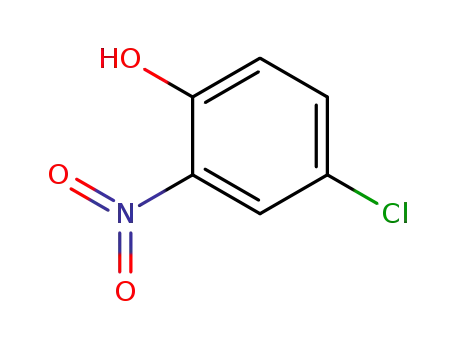
p-chloro-o-nitrophenol
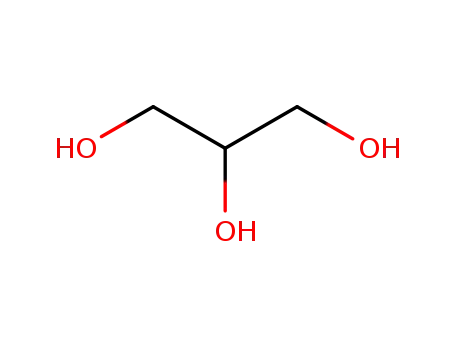
glycerol
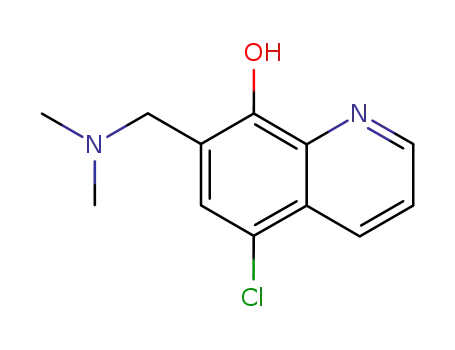
5-chloro-7-dimethylaminomethyl-quinolin-8-ol
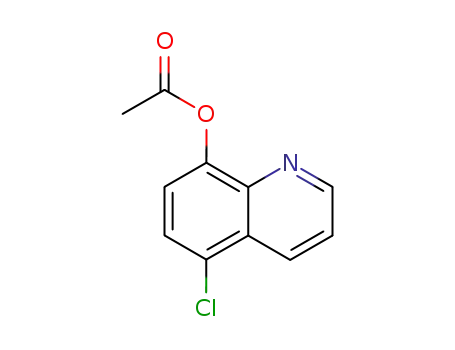
5-chloroquinoline-8-yl acetate
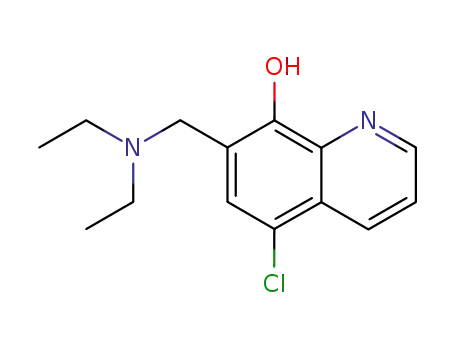
5-chloro-7-((diethylamino)methyl)quinolin-8-ol
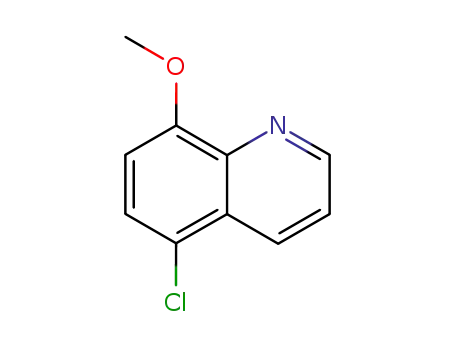
chloro-5 methoxy-8 quinoleine