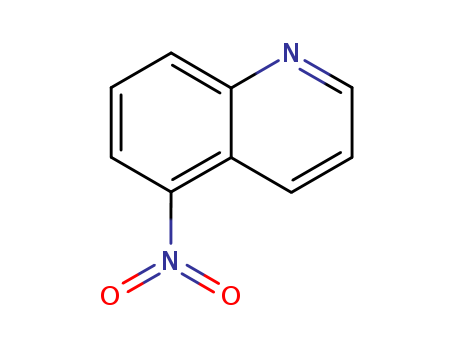

CasNo: 607-34-1
MF: C9H6N2O2
Appearance: white to light yellow crystal powder
|
Safety Profile |
Mutation data reported. Whenheated to decomposition it emits toxic fumes of NOx. Seealso other nitroquinoline entries. |
|
Purification Methods |
Crystallise 5-nitroquinoline from pentane, then from *benzene. The hydrochloride has m 224o and the picrate has m 206o, 214o(from MeOH). [Beilstein 20 H 371, 20 II 235, 20 III/IV 3397.] |
InChI:InChI=1/C9H6N2O2/c12-11(13)9-5-1-4-8-7(9)3-2-6-10-8/h1-6H
Deoxygenation of heteroaromatic N-oxides...
To study the antiparasitic 8-nitroquinol...
An efficient strategy for the deoxygenat...
Deoxygenation of various types of N-oxid...
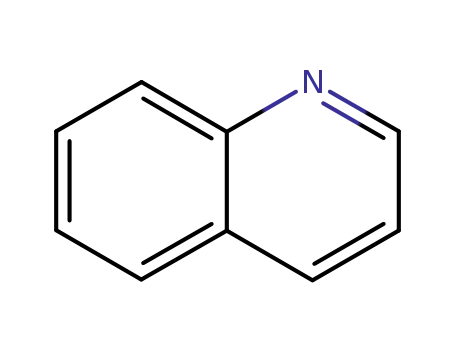
quinoline

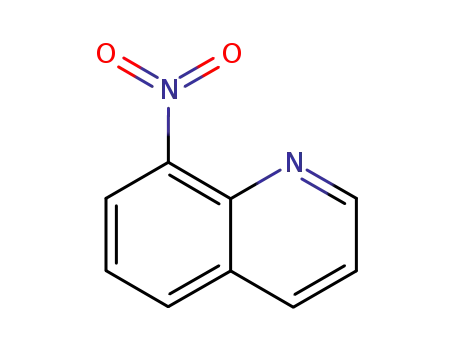
8-nitroquinoline

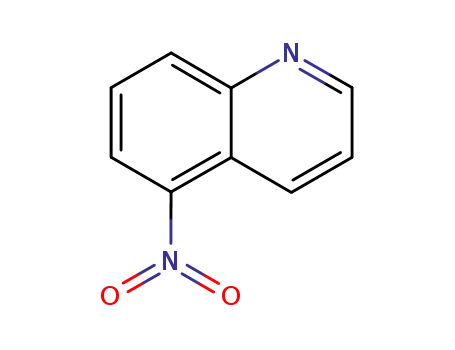
5-nitroquinoline
| Conditions | Yield |
|---|---|
|
quinoline;
With
sulfuric acid; nitric acid;
at 20 ℃;
for 1h;
With
sodium carbonate;
In
water;
|
34% 38% |
|
With
sulfuric acid; nitric acid;
In
water;
at 0 - 20 ℃;
for 1h;
|
35% |
|
With
sulfuric acid; nitric acid;
|
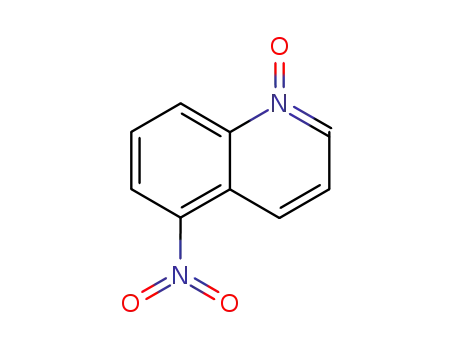
5-nitro-quinoline-1-oxide


5-nitroquinoline
| Conditions | Yield |
|---|---|
|
With
Methyl phenyldiazoacetate; copper(II) bis(trifluoromethanesulfonate);
In
1,2-dichloro-ethane;
at 60 ℃;
for 12h;
Inert atmosphere;
Sealed tube;
Molecular sieve;
|
94% |
|
With
tris(pentafluorophenyl)borate; phenylsilane;
In
dichloromethane;
at 60 ℃;
for 8h;
Inert atmosphere;
Schlenk technique;
Green chemistry;
|
81% |
|
With
lithium tetrafluoroborate;
In
water; acetonitrile;
at 20 ℃;
for 4h;
Electrochemical reaction;
Inert atmosphere;
|
79% |

quinoline
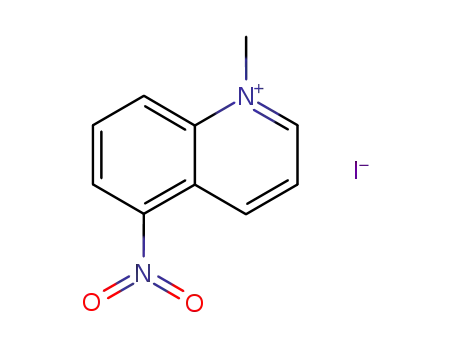
1-methyl-5-nitroquinolin-1-ium iodide
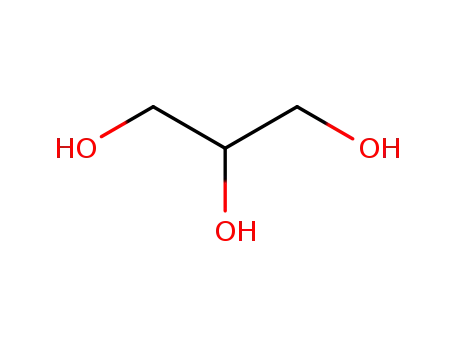
glycerol
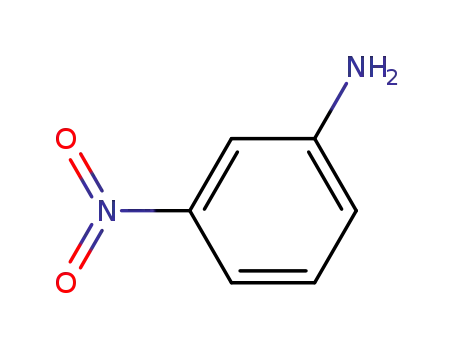
3-nitro-aniline
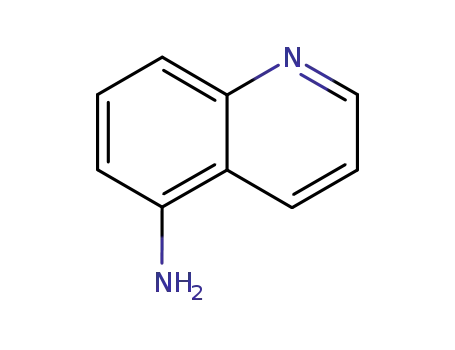
5-Aminoquinoline
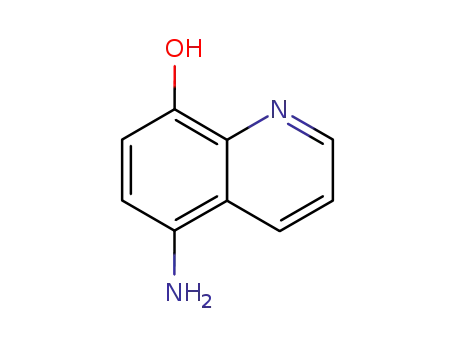
5-amino-8-hydroxyquinoline

5-nitroquinolin-2-ol
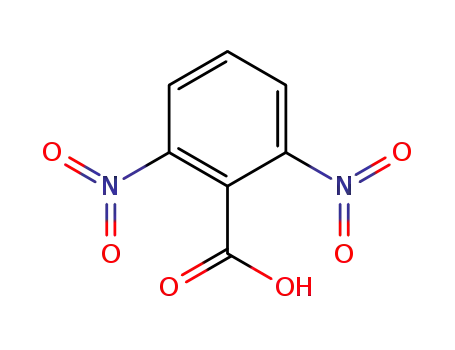
2,6-dinitrobenzoic acid