
CasNo: 580-16-5
MF: C9H7NO
Appearance: white to light yellow crystal powder
|
Definition |
ChEBI: A monohydroxyquinoline that is quinoline substituted by a hydroxy group at position 6. |
|
General Description |
6-Hydroxyquinoline is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline encaged in catalytic Na+-exchanged faujasite zeolites X and Y have been explored by measuring steady-state and picosecond time-resolved spectra. |
InChI:InChI=1/C9H7NO/c11-8-3-4-9-7(6-8)2-1-5-10-9/h1-6,11H
In this study, seventeen novel quinoline...
An efficient and modified Skraup reactio...
Visible-light-driven organic reactions a...
Monomeric active species are very intere...
Devising artificial photoenzymes for abi...
The design and development of an oxime-b...
![1a,7b-dihydrooxireno[2,3-f]quinoline](/upload/2025/3/61cae818-a8cf-4da9-a799-87d36e5b31b1.png)
1a,7b-dihydrooxireno[2,3-f]quinoline

6-hydroxyquinoline


quinolin-5-ol


5,6-trans-dihydroxy-5,6-dihydroquinoline
| Conditions | Yield |
|---|---|
|
With
sodium perchlorate;
In
1,4-dioxane; water;
at 25 ℃;
for 45h;
Title compound not separated from byproducts;
pH 2.37;
|
|
|
With
sodium perchlorate;
In
1,4-dioxane; water;
at 25 ℃;
for 45h;
Rate constant;
Product distribution;
Mechanism;
pH 2.37; other pH: kH - hydronium ion catalyzed, ko - pH-independent, kOH - hydroxide ion catalyzed, pH-rate profiles, pKa;
|
![1a,7b-dihydrooxireno[2,3-f]quinoline](/upload/2025/3/61cae818-a8cf-4da9-a799-87d36e5b31b1.png)
1a,7b-dihydrooxireno[2,3-f]quinoline


6-hydroxyquinoline


quinolin-5-ol
| Conditions | Yield |
|---|---|
|
With
silica gel;
at 110 ℃;
for 0.25h;
isomerization;
|
86% 14% |
|
With
silica gel;
at 110 ℃;
for 0.25h;
Product distribution;
|
quinoline
6-methoxy quinoline
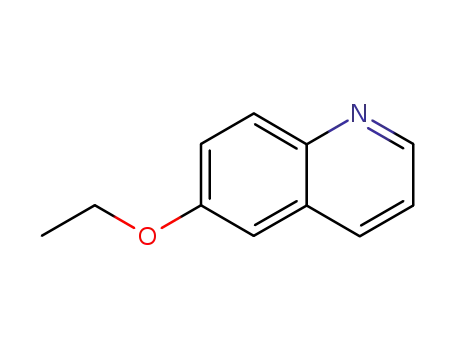
6-ethoxy-quinoline
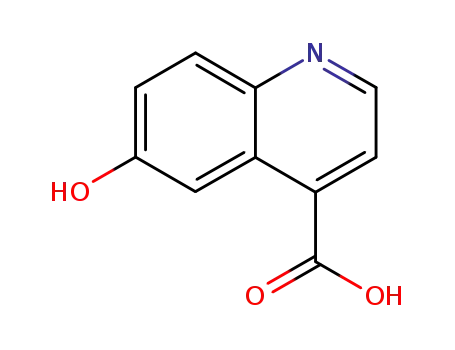
6-hydroxyquinoline-4-carboxylic acid
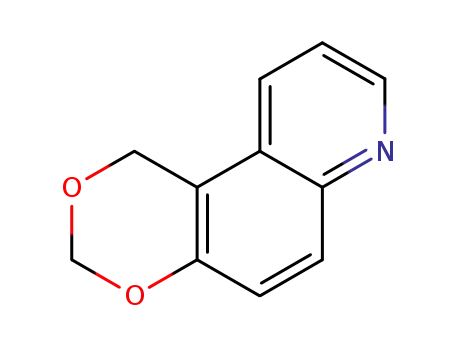
1H-[1,3]dioxino[5,4-f]quinoline
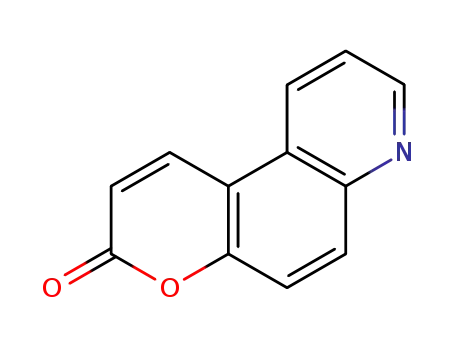
3H-pyrano[3,2-f]quinoline-3-one
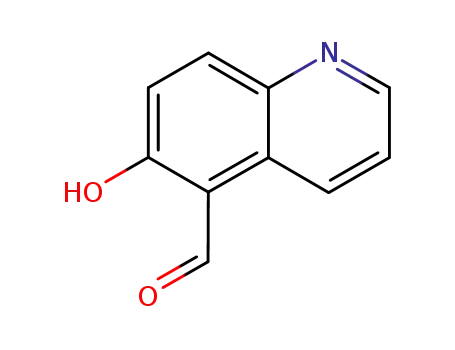
6-hydroxyquinoline-5-carbaldehyde
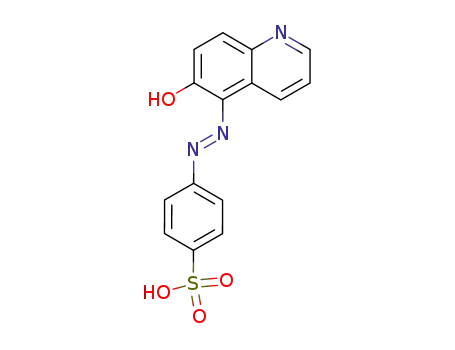
4-(6-hydroxy-quinolin-5-ylazo)-benzenesulfonic acid