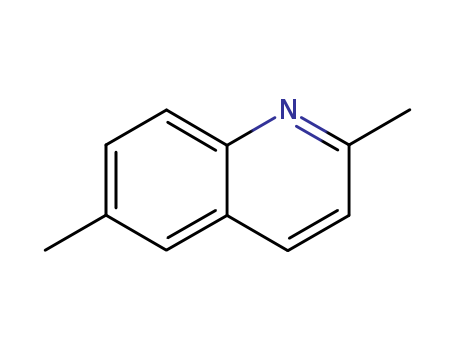

CasNo: 877-43-0
MF: C11H11N
Appearance: white to light yellow crystal powder
|
Biochem/physiol Actions |
2,6-Dimethylquinoline is the chemical constituent present in roots of Peucedantu praeruptorum. It is a potential inhibitor of cytochrome P450 1A2 activity. |
InChI:InChI=1/C11H11N/c1-8-3-6-11-10(7-8)5-4-9(2)12-11/h3-7H,1-2H3
The complete 1H, 13C and 15N NMR assignm...
A combination of NaHSO4.H2O and Na2Cr2O7...
Herein, we disclose a highly chemoselect...
We report herein an unprecedented combin...
The invention relates to a method for pr...
The method takes the aromatic nitro comp...
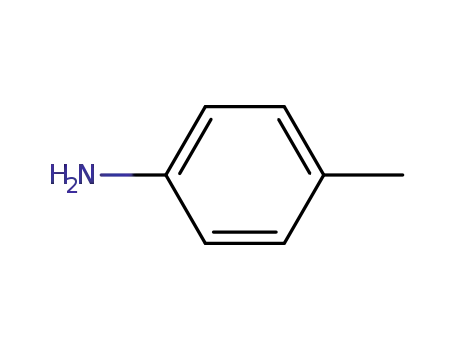
p-toluidine

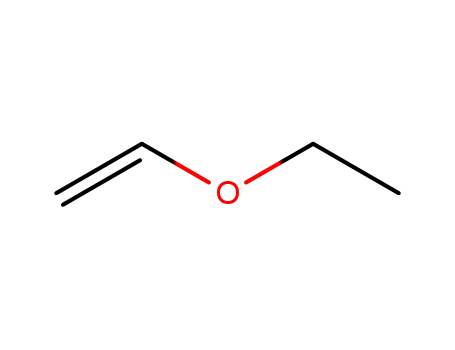
ethyl vinyl ether

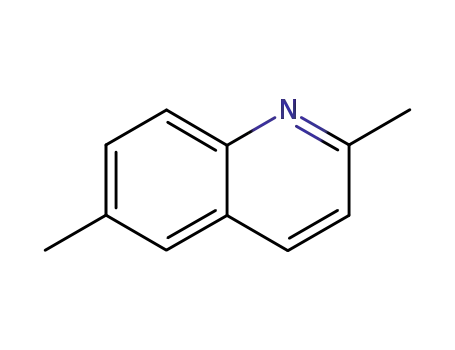
2,6-dimethylquinoline

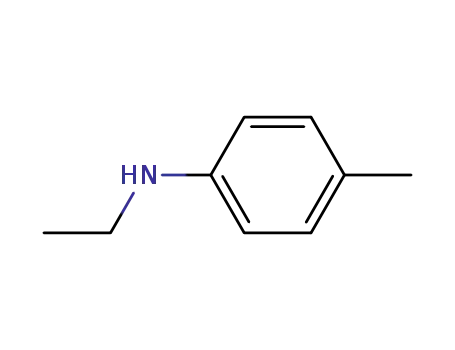
N-ethyl-p-tolylamine
| Conditions | Yield |
|---|---|
|
With
palladium on activated charcoal; palladium dichloride;
In
acetonitrile;
at 80 ℃;
for 24h;
|
82 %Chromat. 12 %Chromat. |
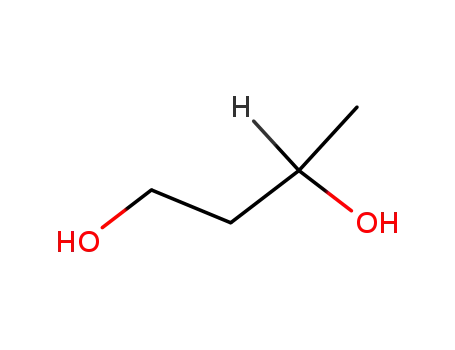
1.3-butanediol

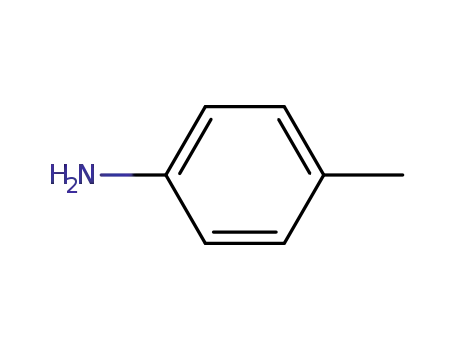
p-toluidine

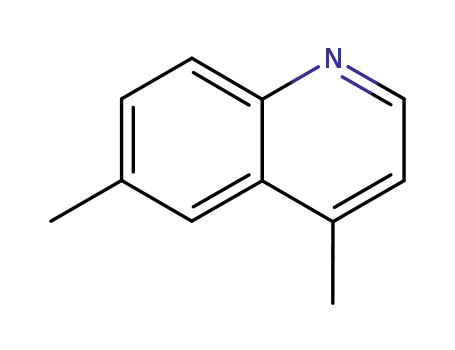
4,6-dimethylquinoline


2,6-dimethylquinoline
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride hexahydrate;
In
tetrachloromethane;
at 150 ℃;
for 8h;
Overall yield = 93 %;
Inert atmosphere;
|
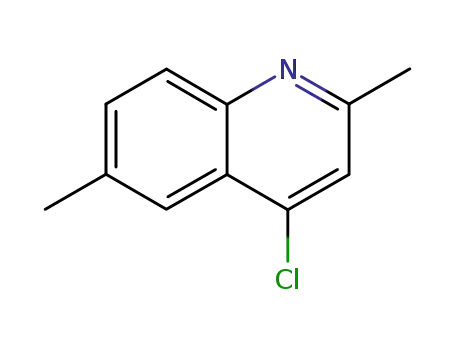
4-chloro-2,6-dimethylquinoline
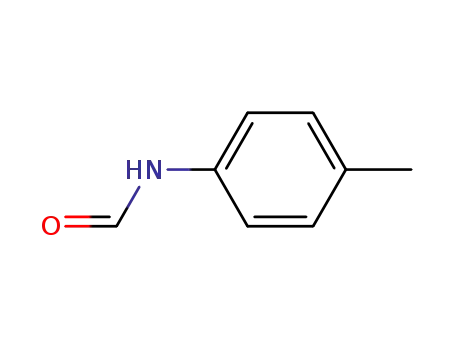
N-(4-methylphenyl)formamide
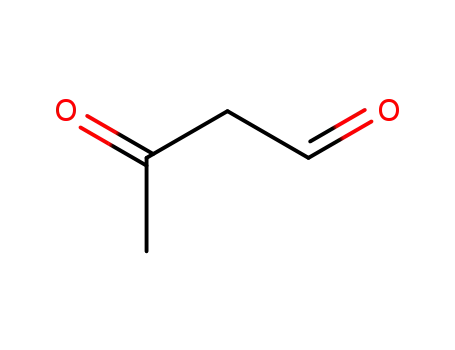
3-oxobutyraldehyde
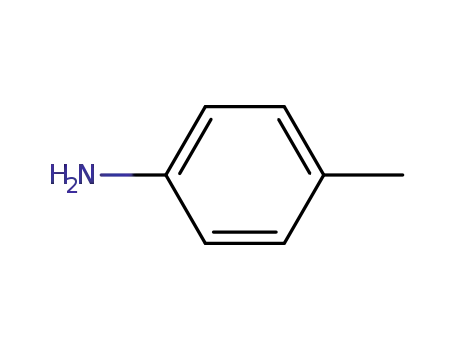
p-toluidine
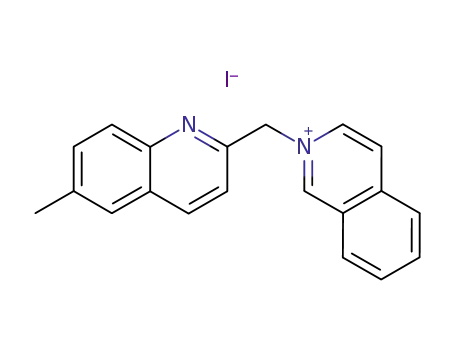
2-(6-methyl-[2]quinolylmethyl)-isoquinolinium; iodide
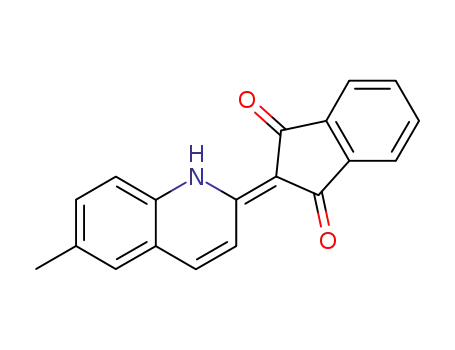
2-(6-methyl-1H-[2]chinolyLiDene)-indane-1,3-dione
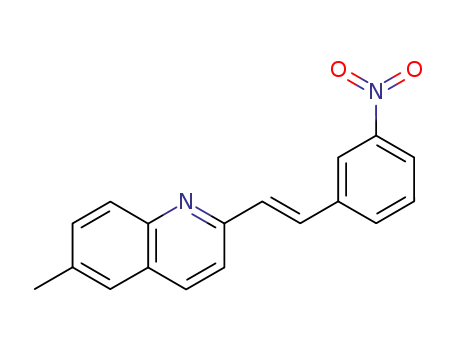
6-methyl-2-(3-nitro-styryl)-quinoline
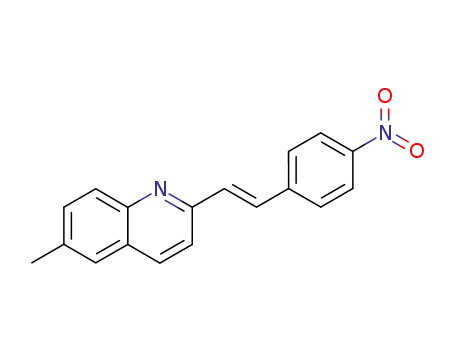
(E)-6-methyl-2-(4-nitrostyryl)quinoline