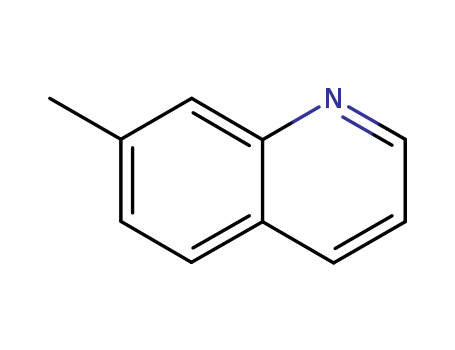

CasNo: 612-60-2
MF: C10H9N
Appearance: white to light yellow crystal powder
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
7-Methylquinoline may be sensitive to exposure to light. May react vigorously with strong oxidizing agents and strong acids . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Fire Hazard |
7-Methylquinoline is combustible. |
|
Purification Methods |
Purify it via its dichromate complex (m 149o, after five recrystallisations from water). [Cumper et al. J Chem Soc 1176 1962, Beilstein 20 III/IV 3497, 20/7 V 402.] |
|
General Description |
7-Methylquinoline is a reagent used in the preparation of diarylmethylpiperazines as potent opioid receptor agonists with improved side effects. Also used in the preparation of novel pyrazine compounds derived from 2-phenylquinolin-7-yl which act as potent insulin-like growth factor-I receptor inhibitors. |
InChI:InChI=1/C10H9N/c1-8-4-5-9-3-2-6-11-10(9)7-8/h2-7H,1H3
The direct dehydrogenation of alkanes is...
Catalytic methods for the aerobic dehydr...
The invention discloses a green preparat...
In this work, catalytic hydrogen transfe...
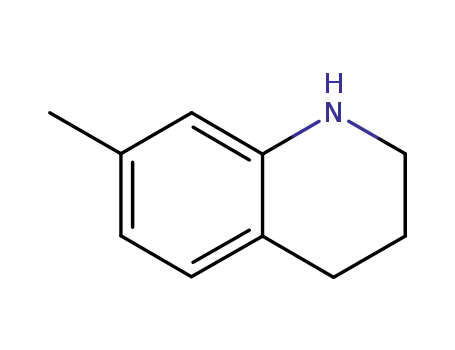
7-methyl-1,2,3,4-tetrahydroquinoline

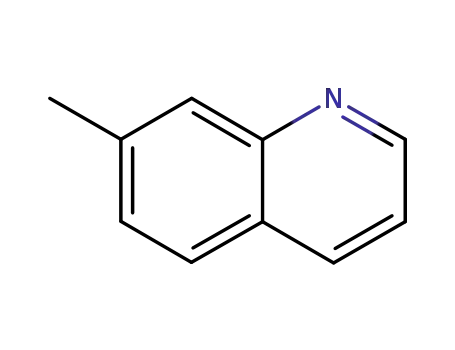
7-methylquinoline
| Conditions | Yield |
|---|---|
|
With
copper(I) oxide; dmap; N-hydroxyphthalimide; oxygen;
In
acetonitrile;
at 120 ℃;
for 12h;
Sealed tube;
|
92% |
|
With
copper(I) oxide; dmap; N-hydroxyphthalimide; oxygen;
In
acetonitrile;
at 120 ℃;
for 12h;
|
92% |
|
With
potassium carbonate;
In
ethanol;
at 20 ℃;
for 18h;
Irradiation;
|
90% |
|
With
cobalt(II) 5,10,15,20-tetraphenylporphyrin; oxygen;
In
N,N-dimethyl-formamide;
for 11h;
|
86% |
|
With
hexagonal boron carbon nitride;
In
water;
at 25 ℃;
for 12h;
Schlenk technique;
Inert atmosphere;
Irradiation;
|
86% |
|
With
tris(bipyridine)ruthenium(II) dichloride hexahydrate; chloropyridinecobaloxime(III);
In
ethanol;
at 30 ℃;
for 6h;
Schlenk technique;
Inert atmosphere;
Irradiation;
|
81% |
|
With
potassium tert-butylate;
In
o-xylene;
at 140 ℃;
for 36h;
Inert atmosphere;
|
80% |
|
With
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrabutylammonium tetrafluoroborate;
In
water; acetonitrile;
at 20 ℃;
for 4h;
Electrochemical reaction;
|
78% |
|
With
dodecacarbonyl-triangulo-triruthenium; iodomesitylene; N,N'-1,2-tetrakis(4-fluorophenyl)ethane-1,2-diimine; caesium carbonate;
In
chlorobenzene;
at 150 ℃;
for 16h;
Inert atmosphere;
Sealed tube;
|
70% |
|
With
platinum; oxygen;
In
methanol;
at 40 ℃;
under 750.075 Torr;
Schlenk technique;
Sealed tube;
|
100 %Chromat. |
|
With
oxygen;
In
1,3,5-trimethyl-benzene;
at 80 ℃;
for 3.5h;
under 760.051 Torr;
|
|
|
With
tert.-butylhydroperoxide;
In
water;
at 20 ℃;
for 18h;
Sealed tube;
|
87 %Spectr. |

7-methyl-1,2,3,4-tetrahydroquinoline

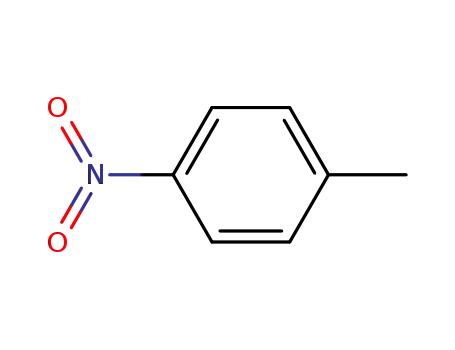
1-methyl-4-nitrobenzene


7-methylquinoline

p-toluidine
| Conditions | Yield |
|---|---|
|
With
nickel-nitrogen-doped carbon framework;
In
water;
at 145 ℃;
for 18h;
Inert atmosphere;
Sealed tube;
Green chemistry;
|
94% 92% |
allyl alcohol
3-Methylcyclohexanone
glycerol
1-amino-3-methylbenzene
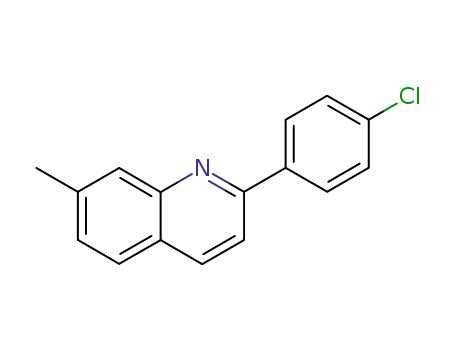
2-(4-chloro-phenyl)-7-methyl-quinoline
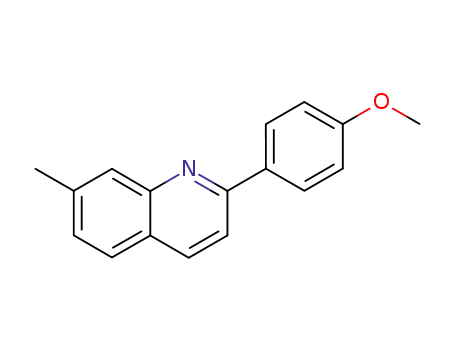
2-(4-methoxy-phenyl)-7-methyl-quinoline
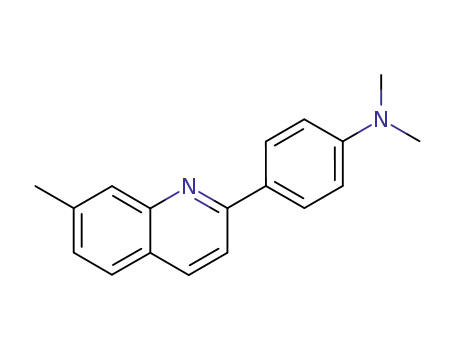
N,N-dimethyl-4-(7-methyl-quinolin-2-yl)-aniline
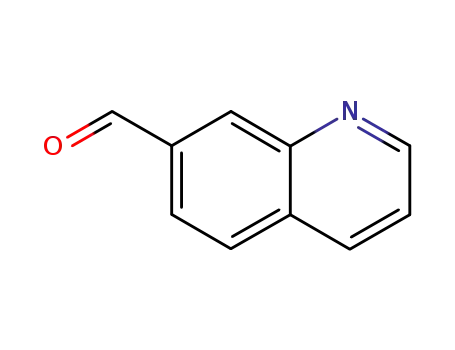
quinoline-7-carbaldehyde