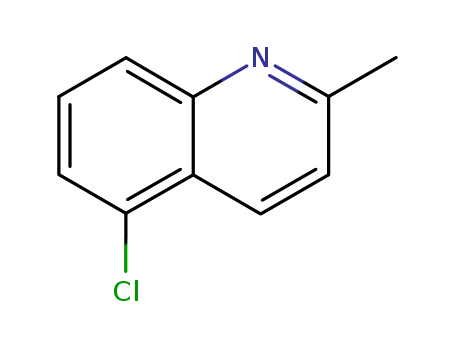

CasNo: 4964-69-6
MF: C10H8 Cl N
|
General Description |
5-Chloroquinaldine is an organic chemical compound with the molecular formula C10H8ClN. It is a yellow solid substance that is soluble in organic solvents and exhibits a strong odor. 5-Chloroquinaldine is primarily used as an intermediate in the production of various agrochemicals and pharmaceuticals. It is commonly used as a building block in the synthesis of pesticides, herbicides, and other crop protection products. Additionally, it is utilized in the manufacturing of pharmaceuticals and research chemicals. 5-Chloroquinaldine is considered to be a hazardous substance and should be handled with caution due to its potential health and environmental risks. |
InChI:InChI=1/C10H8ClN/c1-7-5-6-8-9(11)3-2-4-10(8)12-7/h2-6H,1H3
The synthesis of quinoline derivatives b...
2-, 4-, 6-, 7-, and 8-substituted quinol...
A copper-promoted tandem reaction of a v...
Substituted quinolines were synthesized ...
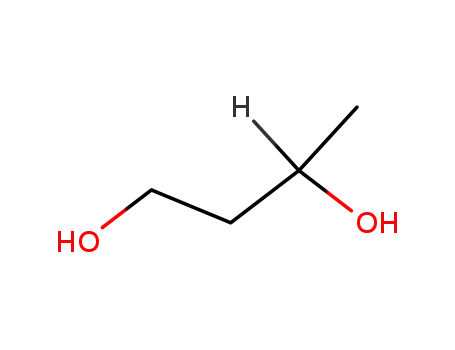
1.3-butanediol

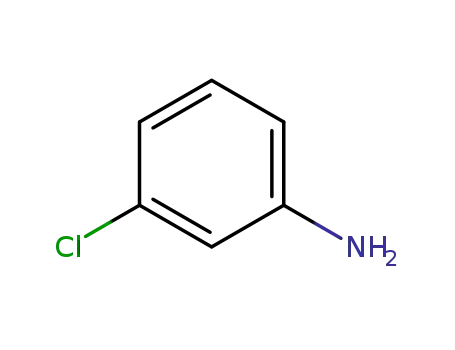
3-chloro-aniline

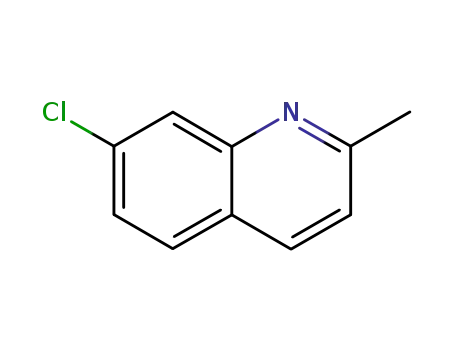
2-methyl-7-chloroquinoline

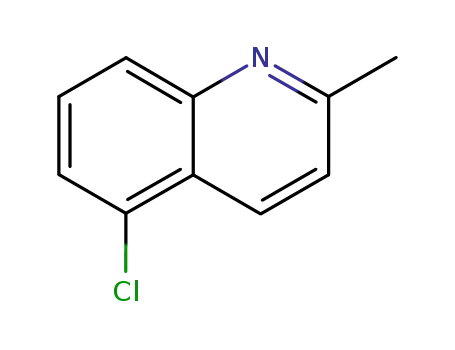
5-chloro-2-methylquinoline

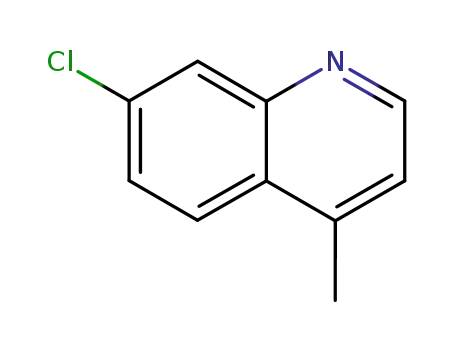
7-chloro-4-methyl-quinoline
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride hexahydrate;
In
tetrachloromethane;
at 150 ℃;
for 8h;
Overall yield = 79 %;
Inert atmosphere;
|
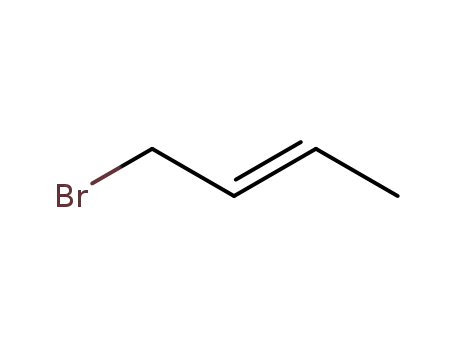
(E)-1-Bromo-2-butene

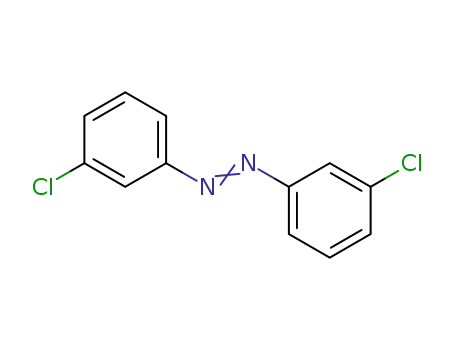
3,3'-dichloroazobenzene


2-methyl-7-chloroquinoline


5-chloro-2-methylquinoline
| Conditions | Yield |
|---|---|
|
With
copper(l) iodide;
In
1,2-dichloro-ethane;
at 120 ℃;
for 12h;
Overall yield = 44 %; Overall yield = 15.6 mg;
Inert atmosphere;
Sealed tube;
|
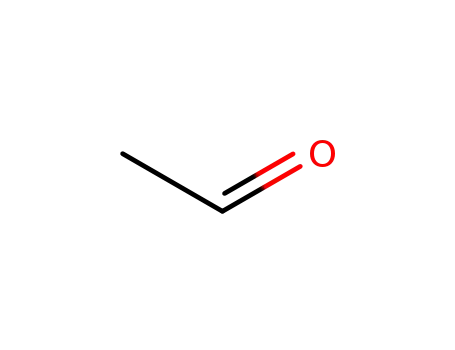
acetaldehyde

3-chloro-aniline
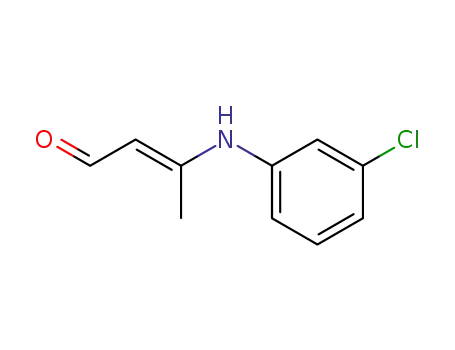
β-(m-chloroanilino)crotonaldehyde
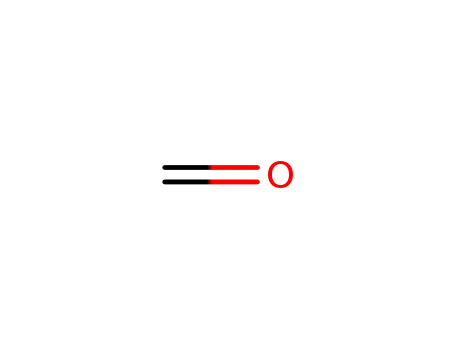
formaldehyd
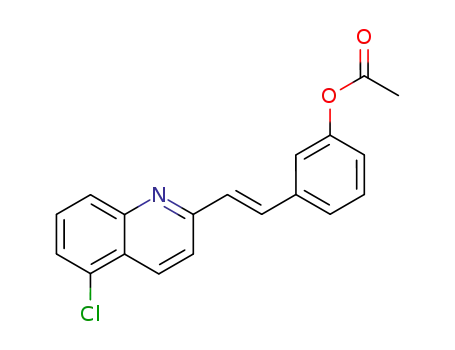
Acetic acid 3-[(E)-2-(5-chloro-quinolin-2-yl)-vinyl]-phenyl ester
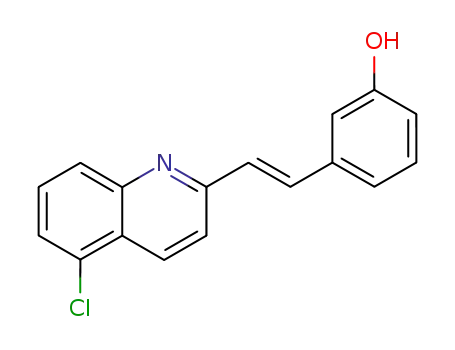
3-[(E)-2-(5-Chloro-quinolin-2-yl)-vinyl]-phenol
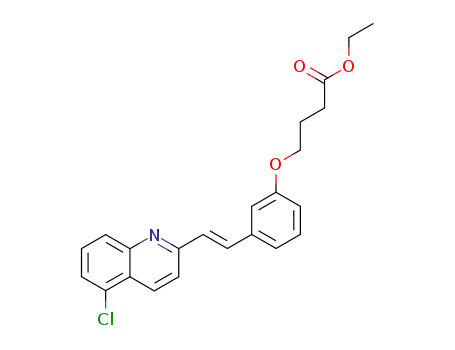
4-{3-[(E)-2-(5-Chloro-quinolin-2-yl)-vinyl]-phenoxy}-butyric acid ethyl ester
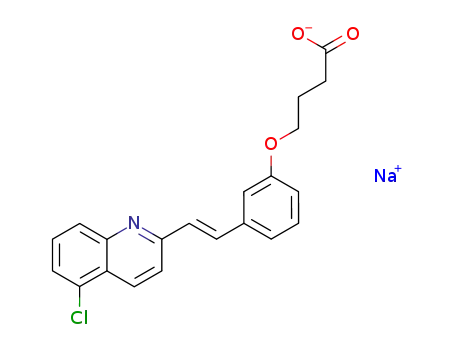
sodium {3-[2-(5-chloro-2-quinolinyl)-(E)-ethenyl]phenoxy}butanoate